Figure 4.
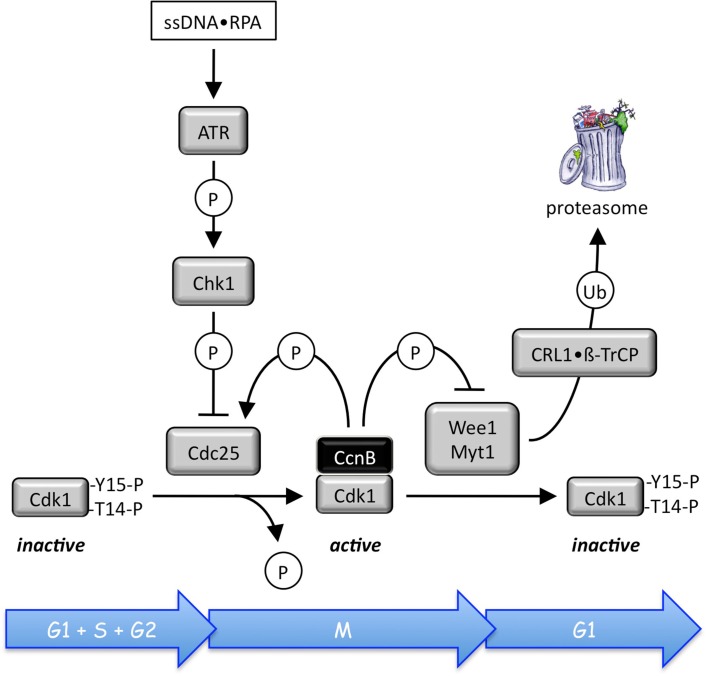
Site specific phosphorylation and dephosphorylation events regulate CDK•cyclin activity (reviewed in Domingo-Sananes et al., 2011). Human CDK•cyclin complexes are maintained in an inactive state through Wee1 phosphorylation of Tyr-15, and Myt1 phosphorylation of both Tyr-15 and Thr-14 adjacent to the CDK catalytic site. For example, prophase begins when the stockpile of Thr-14 and Tyr-15 phosphorylated Cdk1•CcnB is abruptly activated by Cdc25-mediated dephosphorylation of Cdk1. As Cdk1•CcnB activity appears, it catalyzes its own activation by two feedback loops. Wee1 is phosphorylated by Cdk1•CcnB, facilitating its degradation by the ubiquitin ligases CRL1•βTrCP and CRL1•Tome1. Conversely, Cdc25 is activated by multiple CDK phosphorylations. This allows Cdk1•CcnB to convert graded inputs into switch-like, irreversible responses once a critical portion of the enzyme becomes active. In mammalian cells, all three Cdc25 isoforms have been implicated in cell cycle regulation. Cdc25A regulates both G1/S and S/G2 transitions, while Cdc25B and Cdc25C regulate late G2 and mitosis, respectively (Lavecchia et al., 2010).