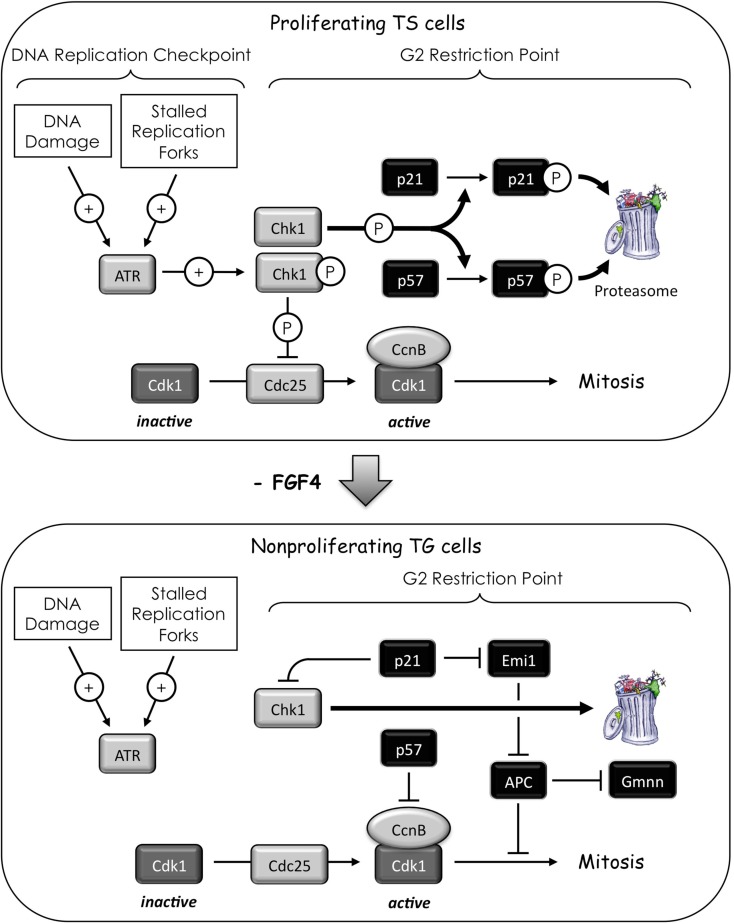
Figure 7.
Activating endocycles in the mouse trophectoderm lineage. Chk1 is the effector kinase for two checkpoints. The “DNA replication checkpoint” prevents premature mitosis in response to DNA damage and stalled replication forks. These events activate (+) the ATR kinase that activates the Chk1 kinase that inhibits (⦹) the CDC25 phosphatase through site specific phosphorylation. Since CDC25 is required to activate Cdk1, inhibition of CDC25 prevents entrance into mitosis. The “G2-restriction point” prevents expression of p57 and p21 in response to mitogen stimulation. In the case of trophoblast stem (TS) cells, the mitogen is FGF4. In the presence of FGF4, TS cells express the p21 and p57 genes, but Chk1 phosphorylates the p21 and p57 proteins, thereby targeting them for degradation by the 26S proteasome. This prevents TS cells from exiting their mitotic cell cycle. In the absence of FGF4, Chk1 protein is degraded, thereby up-regulating p57 and p21. A feedback loop exists in which p21 could sustain suppression of Chk1 expression in trophoblast giant (TG) cells (Gottifredi et al., 2001). Inhibition of Cdk1 by p57 triggers endoreplication and differentiation into TG cells. This event could be facilitated by p21-dependent down-regulation of Emi1, a specific inhibitor of the APC (Lee et al., 2009). The APC targets mitotic cyclins and Geminin for degradation, thereby promoting origin licensing. The absence of Chk1 in TG cells allows p57 protein levels to oscillate. As CcnE levels increase, Cdk2•CcnE will eventually phosphorylate p57 at a CDK-specific site, thereby targeting it for degradation.