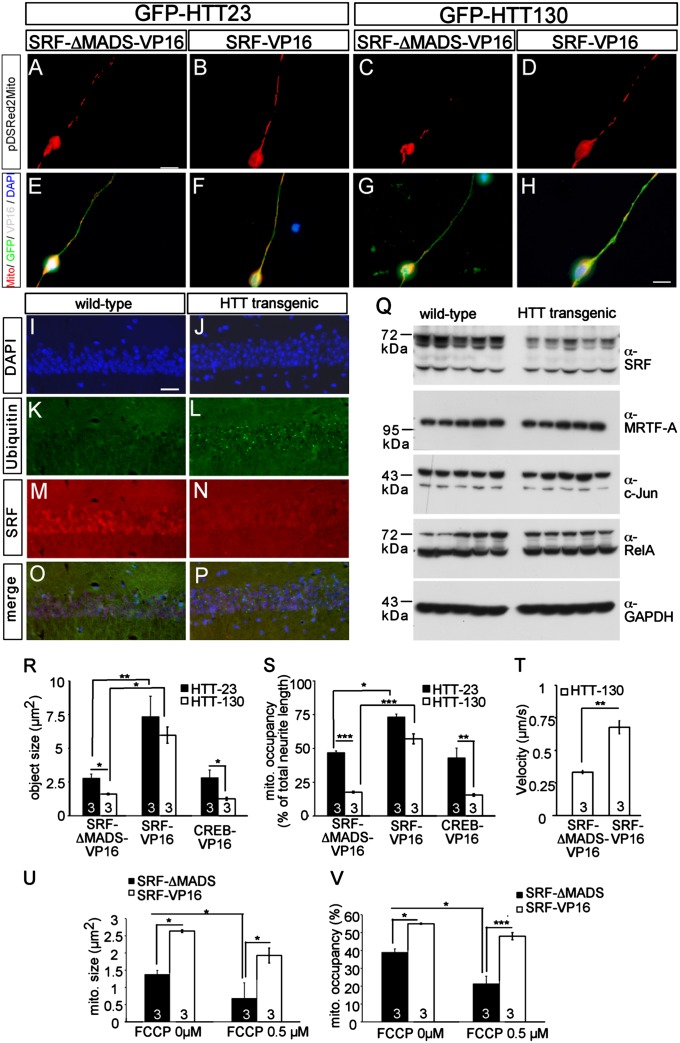
Fig. 4.
SRF-VP16 modulates neurodegeneration-inflicted mitochondrial dynamics. (A–H) In primary neurons, GFP-HTT130, an aggregation-competent huntingtin mutant, induced mitochondrial fragmentation and decreased occupancy (C and G) compared with the control GFP-HTT23 (A and E) in an SRF-ΔMADS-VP16–expressing neuron. (D and H) SRF-VP16 inhibited mitochondrial fragmentation and increased mitochondrial occupancy in the presence of aggregation-competent HTT130. (I–P) SRF (N) is down-regulated in the hippocampal CA1 region of adult huntingtin transgenic mice compared with a nuclear SRF stain in WT mice (M). Antiubiquitin staining depicts huntingtin aggregates in HTT transgenic mice (L) but not in controls (K). (Q) Lysates of hippocampi derived from WT and HTT transgenic mice were blotted for the indicated antibodies. Full-length SRF (approximately 67 kDa), in contrast to other gene regulators (c-Jun, MRTF-A, RelA), is down-regulated in huntingtin transgenic mice. SRF-VP16 but not CREB-VP16 modulated mitochondrial (mito.) dynamics inflicted by HTT130 quantified by object size (R) and occupancy (S). (T) Mitochondrial transport velocity known to be decreased by aggregation-competent HTT130 is almost doubled by SRF-VP16 but not by the SRF control construct (SRF-ΔMADS-VP16). FCCP decreases size (U) and occupancy of mitochondria (V) in WT neurons. SRF-VP16 enhanced mitochondrial size (U) and neurite occupancy (V) in the presence of FCCP. (*P < 0.05; **P < 0.01; ***P < 0.001.) Numbers in bars represent numbers of independent experiments. (Scale bar: A–H, 10 μm; I–P, 20 μm.)