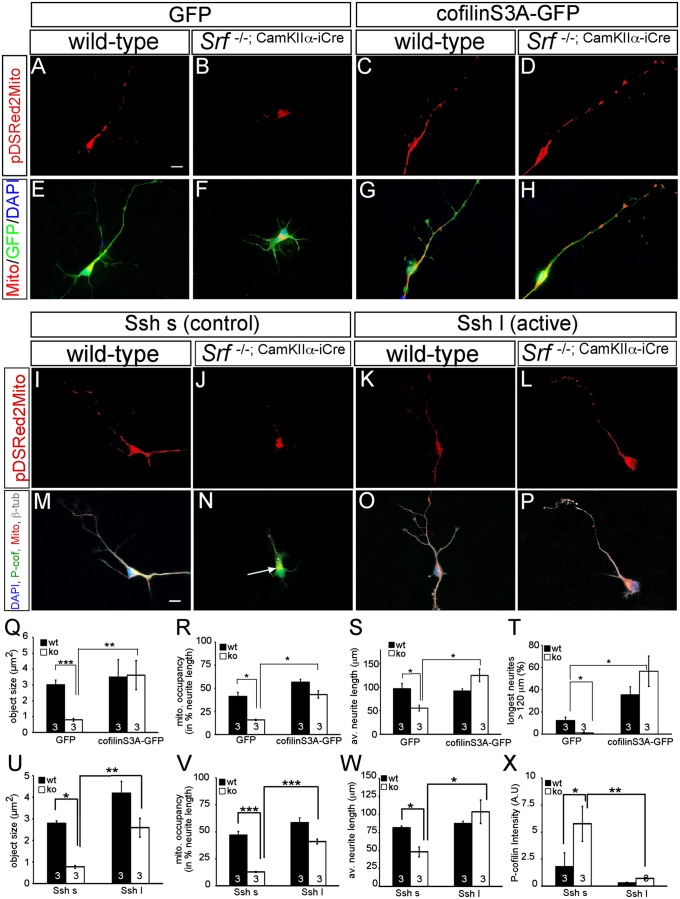
Fig. 6.
Active cofilin and slingshot rescue mitochondrial motility and neurite growth impaired by SRF ablation. In primary neurons, SRF ablation resulted in mitochondrial fragmentation (B and F) compared with WT (A and E) as before (Fig. 2). (C and G) Overexpression of cofilin S3A in WT neurons only slightly enhanced mitochondrial size and neurite occupancy (compare with Q and R). (D and H) Active cofilin S3A rescued impaired mitochondrial dynamics induced by SRF deficiency and elevated neurite growth. (I–P) In WT neurons, active Ssh l (K and O) in comparison to the control Ssh s (I and M) increased mitochondrial size and neurite occupancy, although not statistically significantly (compare with U and V). (L and P) Active Ssh enhanced mitochondrial size and occupancy as well as neurite growth in Srf mutant neurons. In addition, Ssh l (P) suppressed cofilin phosphorylation in SRF-deficient neurons (compare N with P). Cofilin S3A rescued mitochondrial (mito.) size (Q) and neurite occupancy (R) in Srf mutants. (S and T) SRF-deficient neurons expressing cofilin S3A achieved neurite length comparable to WT neurons (S). av., average; ko, knockout; wt, wild type. (T) Cofilin S3A also enhanced neurite growth in WT neurons. Active Ssh l rescued mitochondrial size (U), neurite occupancy (V), and neurite length (W) in Srf mutant neurons. (X) Ssh reduced P-cofilin in WT and more strongly in Srf mutants. (*P < 0.05; **P < 0.01; ***P < 0.001.) Numbers in bars represent numbers of independent experiments. (Scale bar: A–P, 10 μm.)