Fig. 2.
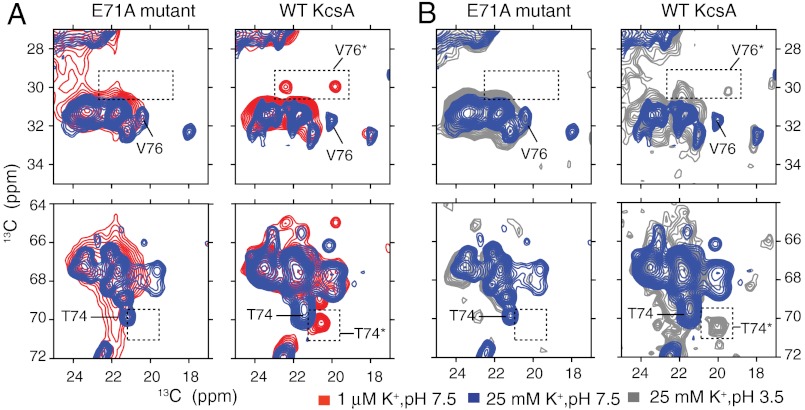
Sections of 13C–13C 2D spectra of both WT-KcsA and the inactivation-resistant mutant E71A are shown with key markers highlighted. Samples are in 9∶1 DOPE∶DOPS lipid bilayers and measured at 0–10 °C. (A) Spectra at pH 7.5 and two K+ concentrations, approximately 1 μM (red) and 25 mM (blue), show that the characteristic collapsed-state chemical shifts (V76* and T74*) are detected for the WT protein at low K+, but not detected for the mutant E71A at low ambient [K+], which instead remains conductive. The absence of the collapsed state for E71A and the presence for the WT channel is reproduced at several markers, including V76 Cβ-Cγ (30 ppm, 19.5 ppm) and T74 Cβ-Cγ (70.3 ppm, 20.5 ppm). (B) Spectra at high [K+] and two pH conditions, 3.5 (gray) and 7.5 (blue), are shown for both WT-KcsA and the mutant E71A. The WT low-pH spectra show some signature collapsed-state shifts suggesting that at low pH, a state similar to the collapsed state is sampled. These data show that, unlike the WT, the E71A filter does not collapse at low K+ or at low pH.