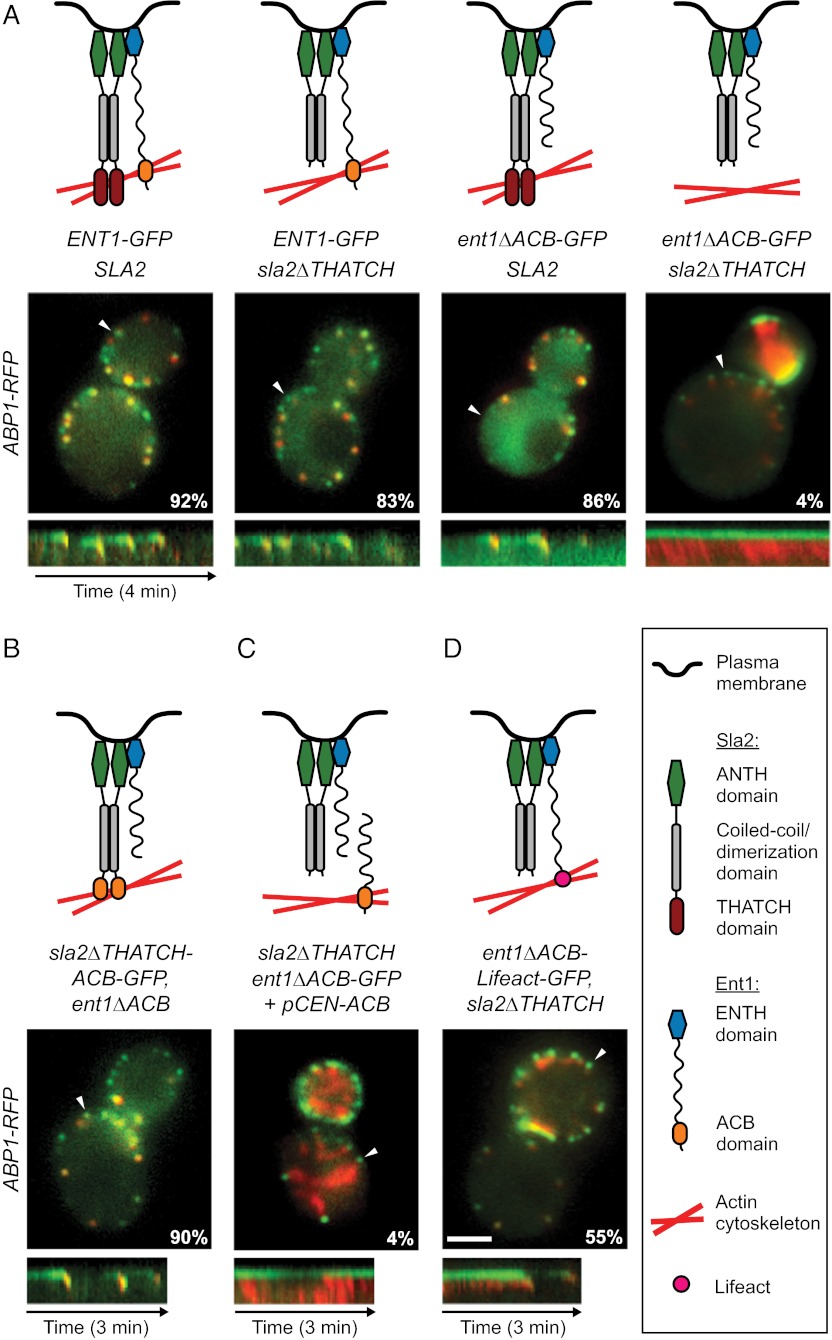
Fig. 5.
Ent1 ACB and Sla2 THATCH domains bind the actin cytoskeleton redundantly. (A) (Top) Schematic models of function of individual Ent1-Sla2 combinations. (Middle) Merged images of four strains expressing Sla2 or Sla2∆THATCH in combination with Ent1-GFP or Ent1∆ACB-GFP together with Abp1-RFP as an actin marker. The percentage of endocytic patches internalized during 4-min movies is shown for each strain. (Bottom) Kymographs of Ent1-GFP or Ent1∆ACB-GFP and Abp1-RFP from sites indicated by arrowheads in the merged images. (B) Sla2∆THATCH-ACB chimeric protein couples the membrane and the actin cytoskeleton. Sla2∆THATCH-ACB-GFP fusion protein was expressed in ent1∆ACB strain together with Abp1-RFP. The model of chimera function (Top), the merged image and percentage of internalized endocytic patches (Middle), and kymograph (Bottom) are shown as in A. (C) Expression of Ent1 ENTH and ACB domains in trans is not sufficient to couple the actin cytoskeleton to the membrane. Ent1(294–454) was expressed in ent1∆ACB-GFP sla2∆THATCH ABP1-RFP strain from a plasmid. The model of Ent1-Sla2 function (Top), the merged image and the percentage of internalized patches (Middle), and kymograph (Bottom) are shown as in A. (D) The Ent1 ACB domain can be replaced by the heterologous actin-binding domain Lifeact for membrane–actin coupling. The Ent1∆ACB-Lifeact-GFP fusion protein was expressed in the sla2∆THATCH strain together with Abp1-RFP. The model of chimera function (Top), the merged image and the percentage of internalized endocytic patches (Middle), and kymograph (Bottom) are shown as in A. (Scale bar: 2 μm.)