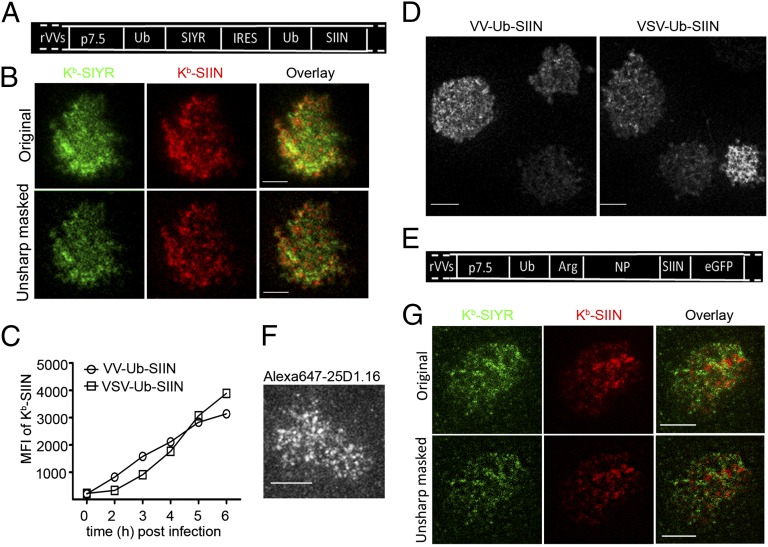
Fig. 3.
Characterizing compartmentalized presentation. (A) Ub-liberated SIYR or SIIN peptides were expressed by VV from a single mRNA with an IRES under the p7.5 VV promoter as indicated. (B) Because of low levels of SIINFEKL expression from the IRES, it was necessary to treat l-Kb cells for 3 d with IFN-γ to increase antigen presentation. Six hours after infecting with the VV-Ub-SIYR-IRES-Ub-SIIN (MOI = 1) cells were stained with Alexa488-2Cm67 and Alexa568-25D1.16 Fab, followed by secondary staining of the Kb–SIIN complexes with Dylight549-conjugated goat anti-mouse IgG Fab to enhance the signal in the corresponding channel. (C) Expression of surface Kb–SIIN between VV-Ub-SIIN and VSV-Ub-SIIN infections. At indicated times p.i., we determined relative levels of surface Kb–SIIN complexes via flow cytometry after Alexa647-25D1.16 staining. (D) Kb–SIIN generated from recombinant vesicular stomatitis virus clusters on the cell surface. Four hours p.i. with either VV-Ub-SIIN or VSV-Ub-SIIN, we visualized surface Kb–SIIN complexes with Alexa647-25D1.16 Fab in live TIRF. (E) Construct used to visualize SIIN presentation from a full-length protein consisting of Ub-Arg-NP-SIIN-eGFP. Due to rapid N-end rule degradation, Kb–SIIN complexes are generated at 3× the rate relative to NP-SIIN-GFP or ovalbumin itself. (F) Six hours p.i. VV-Ub-Arg-NP-SIIN-eGFP, l-Kb cells were labeled live with Alexa647-conjugated 25D1.16 Fab and visualized live with TIRF. (G) Six hours after coinfection with VV-Ub-Arg-NP-SIIN and VV-Ub-Arg-NP-SIYR, l-Kb cells were stained live with a mixture of directly conjugated monovalent reagents Alexa488-conjugated 2Cm67 and Alexa647-conjugated 25D1.16. Stained cells were visualized live with dual-color TIRF.