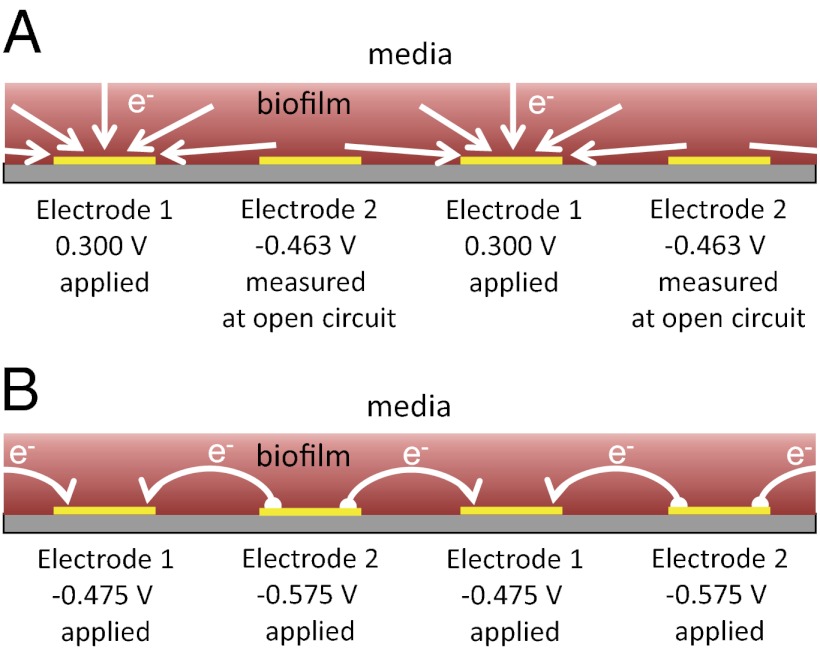
Fig. 2.
Schematic depiction of a cross-section of biofilm-coated IDA. (A) Anode/open circuit experiment in which electrode 1 is used as an anode that collects electrons coupled to cellular oxidation of acetate throughout the biofilm, whereas electrode 2 is at open circuit and therefore, does not accept electrons; however, it is used to measure oxidation state of the biofilm in the vicinity of electrode 2. White arrows indicate flux of the electrons to microelectrode bands comprising electrode 1 coupled to cellular oxidation of acetate throughout the biofilm continuously supplied by diffusion from adjacent media. A specific case is shown, in which the potential applied to electrode 1 is +0.300 V and the open circuit potential measured at electrode 2 is −0.463 V based on the results depicted in Fig. 3B. In this case, while the biofilm is fully oxidized in vicinity of electrode 1, it is only 44% oxidized in vicinity of electrode 2 (Fig. 3B). (B) Electrochemical gate experiment in which different potentials are applied to electrodes 1 and 2 while maintaining a constant potential offset between the electrodes, resulting in electron transport through the biofilm from the more negative electrode (electron source is electrode 2) to the more positive electrode (electron drain is Electron 1), which is indicated by white arrows. A specific case is shown, in which the potential applied to electrode 1 is −0.475 V and the potential applied to electrode 2 is −0.575 V, resulting in the largest conducted current based on the results depicted in Fig. 5A. Unless otherwise noted, potentials are vs. Ag/AgCl.