Abstract
The transcription factor encoded by the E-twenty-six (ETS)-related gene, ERG, is an essential regulator of hematopoietic stem cell function and a potent human oncoprotein. Enforced expression of ERG in murine hematopoietic cells leads to the development of a well-characterized lymphoid leukemia and a less well-defined non lymphoid disease. To clarify the latter, we generated murine bone marrow chimeras with enforced Erg expression in engrafted hematopoietic progenitor cells. As expected, these mice developed lymphoid leukemia. However, the previously reported non lymphoid disease that developed was shown to be a uniform, transplantable leukemia with both erythroid and megakaryocytic characteristics. In vivo, this disease had the overall appearance of an erythroleukemia, with an accumulation of immature erythroblasts that infiltrated the bone marrow, spleen, liver, and lung. However, when stimulated in vitro, leukemic cell clones exhibited both erythroid and megakaryocytic differentiation, suggesting that transformation occurred in a bipotential progenitor. Thus, in mice, Erg overexpression induces the development of not only lymphoid leukemia but also erythro-megakaryocytic leukemia.
Keywords: oncogenesis, hematopoiesis
ETS-related gene (ERG), a member of the E-twenty-six (ETS) family of transcription factors, is a critical regulator of hematopoiesis and a potent human oncoprotein. In mice, correct Erg gene dosage is critical for the maintenance of hematopoietic stem cell (HSC) function (1–3). Mice homozygous for the loss-of-function ErgMld2 mutation die at midgestation, with a profound defect in definitive hematopoiesis (3). Analysis of embryo chimeras has revealed that although Erg is dispensable for HSC formation and specification, it is essential for HSC self-renewal and, thus, for maintenance of definitive hematopoiesis during development. This function may be mediated, in part, by Erg directly regulating the expression of runt-related transcription factor 1 (Runx1) and GATA-binding protein 2 (Gata2) (2). In the adult, heterozygosity for the ErgMld2 mutation causes a reduction in stem and progenitor cell numbers, which, at steady state, manifests as mild thrombocytopenia (3). During regeneration of transplanted marrow or recovery from myelotoxic stress, however, Erg+/Mld2 HSCs appear to favor committed progenitor cell formation and short-term hematopoietic reconstitution, at the expense of long-term HSC maintenance and self renewal (1).
In humans, ERG is a potent oncogene. In prostate cancer, the ERG gene is fused to the regulatory elements of the androgen responsive transmembrane protease, serine 2 (TMPRSS2) gene in more than 50% of cases (4). This fusion results in androgen-induced ERG overexpression in prostate epithelium, which, in mouse models, has been shown to induce development of precancerous prostatic intraepithelial neoplasia (5, 6). ERG is also involved in chromosomal translocations with members of the TET (TLS, EWS and TAF15) subfamily of RNA-binding proteins in 5–10% of Ewing sarcoma and rare cases of acute myeloid leukemia (AML) (7, 8).
In addition to its presence in chromosomal translocations, overexpression of ERG has been associated with a poor prognosis in cytogenetically normal AML and T-cell acute lymphoblastic leukemia (T-ALL) (9, 10), and trisomy of ERG has been strongly implicated in the development of hematological malignancy in Down syndrome (11, 12). Approximately 10% of children with Down syndrome are born with a transient myeloproliferative disorder (TMD), which, in 30% of cases, transforms into acute megakaryocytic leukemia (AMKL) within the first few years of life (13, 14). ERG is highly expressed in cell lines derived from Down syndrome leukemias, as well as sporadic AMKLs, and increased expression has been shown to promote megakaryopoiesis in vitro (12, 15). Combined with its presence in the Down syndrome critical region on chromosome 21, these data have suggested a possible role for ERG in Down syndrome AMKL development. This is supported by recent evidence from mouse studies.
The Ts65Dn mouse model of Down syndrome develops a myeloproliferative disorder (MPD) characterized by increased megakaryocytopoiesis, progressive thrombocytosis, and extramedullary hematopoiesis in the spleen (16). We recently reported that reducing the functional Erg copy number to disomy in this mouse model, by crossing it onto the Erg+/Mld2 heterozygote background, resolved the MPD (11). These data suggest that trisomy of ERG contributes to TMD development in Down syndrome, where it likely cooperates with other promegakaryocytic genes in the Down syndrome critical region, such as DYRK1A [dual-specificity tyrosine-(Y)-phosphorylation regulated kinase 1A] (17). In addition, Malinge et al. have demonstrated that trisomy of 33 human chromosome 21 orthologs plus two additional genetic events, mutation in GATA1 and overexpression of the myeloproliferative leukemia virus oncogene (MPL) W515L mutant protein, resulted in progression to AMKL in the Ts1Rhr mouse model of Down syndrome (17). Thus, although trisomy of ERG appears to be important for TMD development in Down syndrome, additional cooperating genetic events are likely required for AMKL development.
In mice, overexpression of ERG in hematopoietic cells rapidly induces the development of leukemia. Using either a vav promoter–driven ERG transgenic approach, or retroviral-mediated ERG overexpression in bone marrow transplant mice, two separate studies have described the development of ERG-induced T-cell leukemias. Both studies also demonstrated that acquired mutations in Notch1 were a common cooperating event (18, 19). In addition, retroviral-mediated ERG overexpression in fetal liver cells (FLCs) or post–5-fluorouracil (5FU) bone marrow has been reported to induce development of a non lymphoid disease in bone marrow transplant mice. The nature of this disease, however, remains unclear. One group described an erythroid hyperplasia (18), whereas another reported the development of an AMKL (20).
To clarify the nature of the ERG-induced non lymphoid disease, we generated murine bone marrow chimeras with enforced expression of Erg in engrafted hematopoietic progenitor cells. As expected, Erg overexpression induced the development of both a lymphoid leukemia and a non lymphoid disease. The nonlymphoid disease presented as an erythroleukemia in vivo, whereas in vitro, the clonogenic leukemic cells exhibited both erythroid and megakaryocytic potential. These data suggest that the two previously reported ERG-induced non lymphoid diseases may actually be different manifestations of the same condition. We propose that enforced expression of Erg results in development of a erythro-megakaryocytic leukemia.
Results
Erg Induces Development of Lymphoid and Non Lymphoid Leukemias in Mice.
FLCs from embryonic day (E)12.5–E13.5 fetuses, or bone marrow cells from adult mice treated with 5FU, were transduced with a MSCV-Erg-IRES-mCherry or MSCV-IRES-mCherry control retrovirus (Fig. S1). Mice transplanted with Erg-transduced FLCs developed leukemia with a median latency of 150 d following sublethal irradiation (550 rad) and 80 d following lethal irradiation (2× 550 rad). Mice transplanted with post-5FU Erg-transduced adult bone marrow developed leukemia with a median latency of 119 d following sublethal irradiation (Fig. 1A).
Fig. 1.
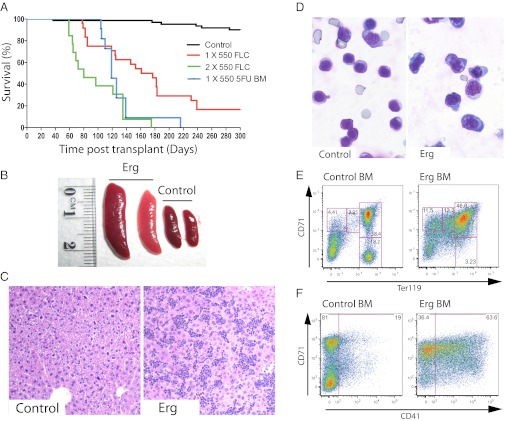
Enforced expression of Erg induces development of leukemia in mice. (A) Survival of murine bone marrow chimeras with enforced expression of Erg in hematopoietic cells. Sublethally irradiated mice were transplanted with hematopoietic cells transduced with empty vector control (black line), FLCs transduced with Erg (red line), or post-5FU bone marrow cells transduced with Erg (blue line). Lethally irradiated mice were transplanted with FLCs transduced with Erg (green line). Mice receiving lethal irradiation succumbed to leukemia more rapidly than sublethally irradiated mice. (B) Bone marrow chimeras that had received Erg-transduced hematopoietic cells, and did not develop lymphoid leukemia, developed enlarged spleens (Erg) compared with mice that had received empty vector–transduced cells (Control). (C) The livers of these mice (Erg) displayed heavy infiltration by leukemic cells, compared with healthy control mice (Control). Representative sections taken at 100× magnification are shown. (D) May–Grunwald–Giemsa staining of representative cytocentrifuge preparations of spleen cells is shown. Cytological analysis of spleen cells from leukemic mice (Erg) revealed a predominance of nucleated erythroid cells with basophilic staining of the cytoplasm, compared with control mice (Control). (E) Flow cytometric analysis of erythroid development in the bone marrow. Normal erythroid development was observed in control mice (Control BM). Erythroid development in leukemic mice (Erg BM) was severely perturbed, with a buildup of mCherry+ cells expressing high levels of CD71 and increasing levels of Ter119. (F) A subset of the mCherry+ leukemic erythroblast cells also expressed the megakaryocytic marker CD41 (Erg BM). This was not observed in control mice (Control BM).
Consistent with previous reports (18, 19), we found that a subset of mice developed classic T-cell leukemias. Disease incidence was 100% in sublethally irradiated recipients of post-5FU bone marrow, whereas in those receiving FLCs, only 60% of sublethally and 30% of lethally irradiated mice developed T-cell leukemia. Histopathologic and flow cytometric analysis of these moribund animals revealed an altered thymic population comprising more than 80% mCherry+ cells with an abnormal CD4/CD8 expression profile (Fig. S2).
Strikingly, we also observed a disease involving hepatosplenomegaly in 100% of lethally irradiated and 50% of sublethally irradiated recipients of FLCs, and 30% of sublethally irradiated recipients of post-5FU bone marrow. Moribund mice, with no histologically evident concomitant lymphoid leukemia, exhibited a red/gray enlarged spleen [762 ± 444 mg (n = 11) versus 72 ± 12 mg (n = 5) in control mice; P < 0.003] and an enlarged pale liver (2,176 ± 880 mg versus 1,153 ± 124 mg in control mice, P < 0.02) (Fig. 1B). No changes were observed in body weight or that of any of the other 16 major organs, including the thymus and mesenteric lymph nodes.
In mice with non lymphoid disease, the liver was heavily infiltrated by leukemic cells, which were dispersed uniformly and not concentrated around portal vessels (Fig. 1C). The lung lacked leukemic cell aggregates around bronchi or major vessels but had a dispersed infiltration of leukemic cells that thickened the alveolar walls. In the enlarged spleen, leukemic cells severely disrupted the normal architecture, with the exception of some small surviving lymphoid follicles. Cytocentrifuge and flow cytometric analyses demonstrated that the spleen and bone marrow contained a uniform population of immature erythroblasts (Fig. 1D and Table S1), expressing mCherry, high levels of CD71, and varying levels of Ter119 (Fig. 1E). In some mice, the spleen and bone marrow contained a subset of CD71+ leukemic cells that also expressed the megakaryocytic marker CD41 (Fig. 1F). Small numbers of acetylcholinesterase-positive cells were observed in sections of liver, bone marrow, and spleen from these mice.
To verify that the infiltrating cells in moribund animals were leukemic, we tested the transplantability of the disease by injecting 10 million unfractionated spleen cells i.v. into secondary recipient mice. These transplanted cells rapidly induced development of leukemia in sublethally irradiated recipient mice (43–110 d posttransplant) and more slowly in nonirradiated mice (longer than 140 d posttransplant). Spleen or bone marrow cells from three primary leukemic mice were transplanted into a total of 32 secondary recipients, of which 30 subsequently developed leukemia. The histopathology of all secondary transplant mice analyzed was identical to that of the primary donor mice, and flow cytometric analyses confirmed that the transplanted leukemias were similar to the transplanted leukemic cells.
In Vitro Analysis of Erg-Induced Non Lymphoid Leukemia.
Agar cultures of primary leukemic bone marrow and spleen did not show marked differences in the frequency of progenitor cells relative to control mice, which was unexpected, given the obvious infiltration of leukemic cells in these organs. The only abnormality observed was the presence of multinucleate macrophages in colonies stimulated with granulocyte-macrophage colony stimulating factor (GM-CSF) in leukemic bone marrow cultures (Table S2). Leukemic cells failed to proliferate in agar cultures. However, when cultured in methylcellulose and stimulated by stem cell factor (SCF) plus interleukin 3 (IL-3) plus erythropoietin (EPO), leukemic spleen cells generated large numbers of small colonies after 7 d of incubation. These small colonies increased progressively in size upon continued incubation, and by day 14, many cultures contained colonies of up to 5,000 cells (Fig. 2A). Colony formation was entirely dependent on continued cytokine stimulation, with no colonies developing in unstimulated cultures. EPO was the most active single agent in these cultures, whereas SCF or IL-3 alone or in combination exhibited almost no proliferative activity. Strong synergism was seen, however, when either SCF or IL-3 was combined with EPO (Fig. 2B). In spleen cultures from 3 representative leukemic mice, the mean frequency of colonies per 50,000 cultured cells was 95 ± 15, suggesting that ∼1 in 500 primary leukemic cells were clonogenic. In contrast, these clonogenic cells showed a high degree of self-generation. Approximately 50% of the cells from large day 14 colonies were clonogenic. Recloning the cells from 20 large day 14 colonies from two separate leukemic mice (each colony containing ∼5,000 cells) generated 2,264 ± 1,262 typical daughter colonies.
Fig. 2.
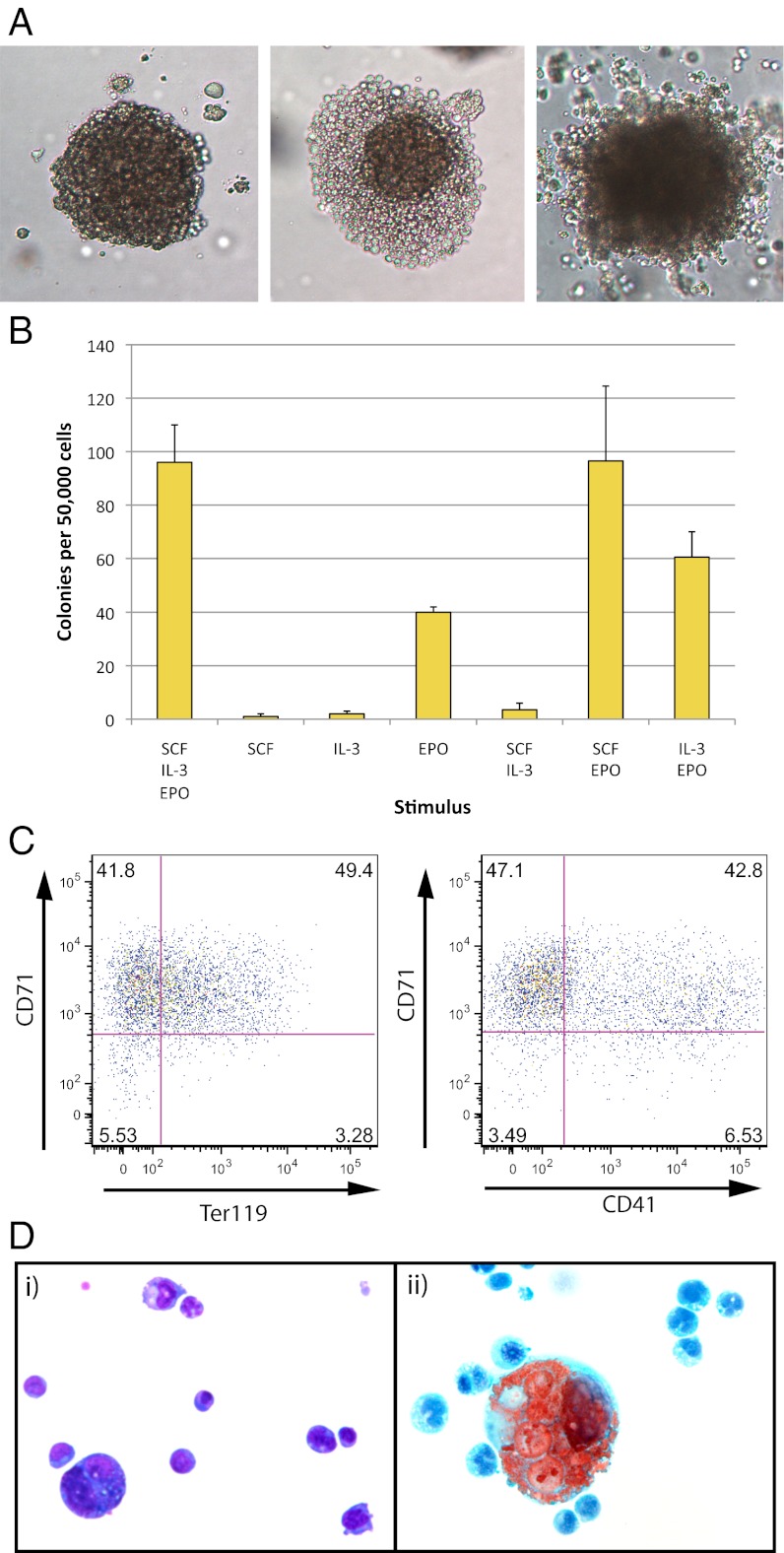
Clonogenic leukemic cells retain the ability to differentiate along both the erythroid and megakaryocytic lineages. (A) Erythroid colonies generated by leukemic cells in methylcellulose. Spleen cells from leukemic mice generated large erythroid colonies when cultured for 14 d in methylcellulose stimulated with IL-3 plus SCF plus EPO. Three representative colonies are shown. (B) Colony formation was completely dependent on continued cytokine stimulation. EPO was the most active single agent; however, SCF and IL-3 were able to synergize with EPO and increase the number of colonies generated. The mean number of colonies per day 14 culture (± SD) from three separate leukemic mice is shown. A total of 50,000 spleen cells was cultured in the different cytokine combinations given along the x axis. (C) Flow cytometric analysis of erythroid colonies. Cells dissociated from pools of day 14 colonies showed the same CD71, Ter119, and CD41 flow cytometric staining pattern as the primary spleen cells. All cells appeared to be immature erythroblast cells (CD71+ Ter119+/−), with a subset also expressing CD41. (D) Individual colonies were dissociated, cytocentrifuged onto duplicate slides, and stained with May–Grunwald–Giemsa (i) or acetylcholinesterase (ii). This analysis revealed that the majority of colonies contained both erythroid (i) and megakaryocytic cells (ii), indicating that the clonogenic leukemic cells were bipotential.
Flow cytometric analysis of mass-harvested day 7 colonies revealed the majority of cells to be immature erythroblasts with an immunophenotype exactly paralleling that of the original leukemic spleen cells in vivo (i.e., mCherry+ CD71+ Ter119+/−) (Fig. 2C). Interestingly, a portion of these CD71+ cells also expressed CD41 (Fig. 2C) in agreement with what was seen in vivo, suggesting that the clonogenic leukemic cells might be bipotential.
To confirm this, 66 individual day 14 colonies grown from 5 separate leukemic mice were cytocentrifuged to generate 2 samples from each colony, 1 for May–Grunwald–Giemsa staining and 1 for acetylcholinesterase staining. All colonies contained dominant populations of immature nucleated erythroblasts, as expected (Fig. 2Di). However, in the matching acetylcholinesterase stained preparations, most colonies also contained some acetylcholinesterase positive cells (Fig. 2Dii). The proportion of acetylcholinesterase-positive cells was quite variable for most colonies but generally below 15% of the total (Fig. S3). Although some cells with the size and morphology of megakaryocytes were evident in the Giemsa-stained preparations, not all such large cells were acetylcholinesterase-positive. Indeed, most of the positive cells were of small size, suggesting either early and/or incomplete megakaryocyte maturation.
Impact of Erg Overexpression on FLC Development and the Preleukemic State.
FLCs transduced with either Erg or control retrovirus showed no significant differences in cell proliferation or survival over the subsequent 3 d in liquid culture stimulated with SCF, interleukin 6 (IL-6), thrombopoietin (TPO), and fms-like tyrosine kinase 3 ligand (Flt3L). Similarly, no significant differences were observed in agar colony formation by mCherry+ or mCherry− cells, which had been flow cytometrically sorted from Erg or control transduced FLC day 3 liquid cultures.
To study the preleukemic period, recipient mice were killed at 2 wk and then weekly from 4 to 7 wk posttransplant. No consistent progression of disease over time was observed. Some mice showed signs of disease at 4 wk posttransplant, and other mice maintained a normal spleen weight and relatively normal erythroid development at 7 wk posttransplant. No consistent differences were observed in the number, frequency, size, or morphology of colonies formed in agar cultures from sorted mCherry+ or mCherry− bone marrow or spleen cells from Erg-transduced or control mice at any time point analyzed.
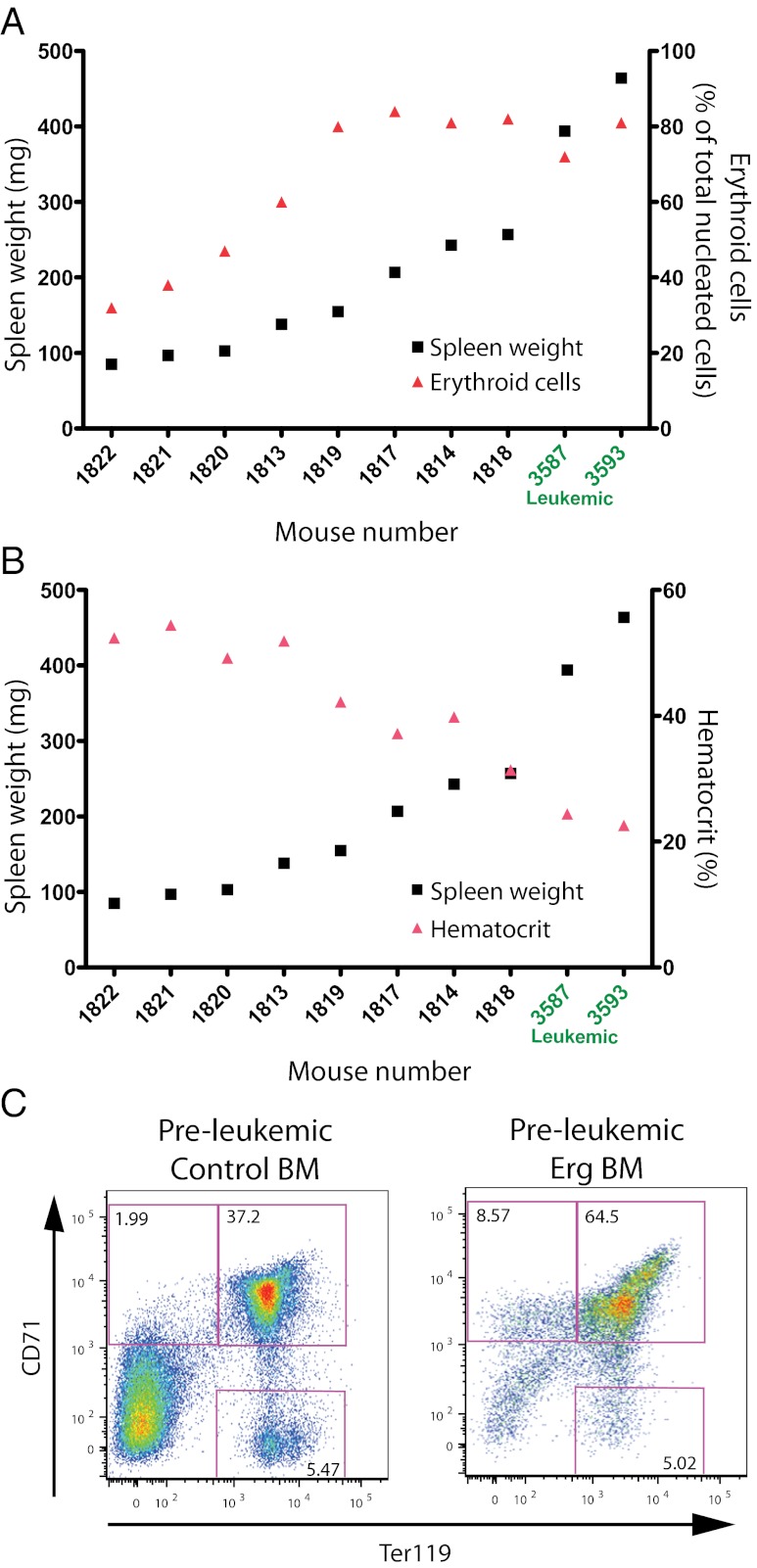
A correlation between increasing spleen weight, the level of erythroid expansion, and severity of anemia was observed (Fig. 3 A and B). This was accompanied by the progressive replacement of the nucleated erythroid compartment with mCherry+ cells. Perturbed erythroid differentiation of the mCherry+ cells was apparent as early as 2 wk posttransplant and was seen in all recipients of Erg-transduced cells analyzed (Fig. 3C). In preleukemic mice, where normal splenic tissue was becoming progressively replaced by expanding populations of mCherry+ immature erythroid cells, two qualitative differences from the leukemic state were observed: (i) an absence of infiltration of the liver by erythroid populations; and (ii) an inability of preleukemic spleen cells to proliferate clonally in methylcellulose cultures.
Fig. 3.
Impact of Erg overexpression on the preleukemic state. (A) Comparison of spleen weight and proportion of erythroid cells in preleukemic mice. In preleukemic mice, increasing spleen weight (black squares) correlated with increasing proportions of splenic erythroid cells (red triangles). Individual mouse numbers are shown on the x axis and are plotted in order of increasing spleen weight. Mice 3587 and 3593 (written in green) were leukemic mice and are shown for comparison. Spleen weight is shown on the left y axis, and proportion of erythroid cells is shown on the right y axis. (B) Comparison of spleen weight and hematocrit in preleukemic mice. In preleukemic mice, the hematocrit (red triangles) progressively decreased as spleen weight (black squares) increased. Individual mouse numbers are shown on the x axis and are plotted in order of increasing spleen weight. Mice 3587 and 3593 were leukemic mice and are shown for comparison. Spleen weight is shown on the left y axis, and hematocrit is shown on the right y axis. (C) Flow cytometric analysis of erythroid development in preleukemic and control bone marrow. Preleukemic mice displayed signs of perturbed erythroid development as early as 2 wk posttransplant. Flow cytometric analysis of bone marrow cells from a representative preleukemic and control mouse pair killed at 6 wk posttransplant is shown.
Discussion
Erg has proved to be a versatile oncogene. This study demonstrates that overexpression of Erg in the hematopoietic compartment of adult mice induces the rapid development of non lymphoid and lymphoid leukemias, sometimes coexisting in the same animal. The lymphoid leukemias in these mice, although not analyzed in depth, appeared identical to those previously described by two other groups (18, 19). In all cases, a classic T-cell leukemia developed in the thymus, with leukemic cells displaying perturbed CD4/CD8 expression.
Given the discrepancy in the published data, we analyzed in more detail the non lymphoid disease induced by Erg. In agreement with Tsuzuki et al. (18), this disease appeared to be largely erythroid in vivo, with cells blocked at the immature erythroblast stage of development. In contrast to the study by Tsuzuki et al., however, we found that spleen cells from moribund animals could transplant disease into recipients. Combined with the heavy infiltration of erythroblasts in the spleen, bone marrow, liver, and, to a lesser extent, the lung in both primary and secondary mice, this confirmed that the disease was leukemic. That Tsuzuki et al. did not observe transplantability might potentially be explained by the difference in the number of primary cells injected, being 10-fold less than in our study or, perhaps more likely, by the cell type transplanted. Tsuzuki et al. used Ter119+ spleen cells, whereas we transplanted unfractionated spleen cells, containing both the (CD71+)Ter119+ and the (CD71+)Ter119− leukemic cell populations. These results suggest that the tumor-sustaining cells reside within the more immature (CD71+)Ter119− population.
In addition to observing the expression of erythroid markers, we also found aberrant expression of the megakaryocytic marker CD41 on a subset of leukemic cells. Although there was little obvious histopathologic evidence of megakaryocytic leukemia in these mice, some small, abnormal acetylcholinesterase-positive cells were visible in tissue sections. Our in vitro studies, on the other hand, clearly demonstrated that the majority of clonogenic leukemic cells could differentiate along both the megakaryocytic and erythroid lineages, albeit in a defective manner. These data, combined with the highly uniform disease phenotype, suggest that Erg overexpression may result in the transformation of an erythroid progenitor cell that retains some megakaryocyte potential. Following transformation, the progeny develop primarily along the erythroid lineage, at least in vivo. The data from Salek-Ardakani et al., however, would suggest that under certain circumstances, this disease can exhibit a more megakaryocytic phenotype (20). Thus, ERG-induced non lymphoid leukemia may be best described as an erythro-megakaryocytic leukemia.
Our data showed that in the murine hematopoietic compartment Erg overexpression favored the transformation of T-cell progenitors and bipotential erythroid-megakaryocytic progenitors. The relative proportion of T-cell and erythro-megakaryocytic leukemias, however, depended on whether FLCs or post-5FU bone marrow cells were transduced, with fewer erythro-megakaryocytic leukemias arising from the latter. This may be simply attributable to the higher proportion of erythroid progenitors in the fetal liver. Despite the skewing in disease proportions, however, the erythro-megakaryocytic leukemias that arose from fetal liver and bone marrow were identical, indicating that the source of the cell of origin did not influence disease phenotype.
We did not observe an immediate effect of Erg overexpression on overall myeloid progenitor cell development in vitro. In fetal liver liquid cultures, cell proliferation and survival were not altered by Erg overexpression, nor was there an impact on clonogenic cell number. Interestingly, erythroid and megakaryocytic progenitor cell development also appeared normal in in vitro clonogenic assays; however, an obvious perturbation of erythroid differentiation in vivo was apparent in all preleukemic mice from as early as 2 wk posttransplant. There were two qualitative differences between preleukemic mice and leukemic mice: specifically, the inability of spleen cells to proliferate in methylcellulose and the inability of transformed cells to infiltrate nonhematopoietic organs. These data, combined with the prolonged disease latency in some mice (longer than 12 mo), would suggest that additional cooperating genetic events are required before overt malignancy develops.
We describe a murine leukemia affecting both the erythroid and megakaryocytic lineages. Erythroleukemia and AMKL are both rare but aggressive subtypes of AML, each accounting for less than 5% of patients diagnosed. The rarity of these diseases, combined with their poor prognosis, has limited progress in elucidating the genetic and biochemical pathways involved. Although ERG is implicated in the development of AMKL, primarily in Down syndrome children, a role for ERG in erythroleukemia has not been described. The ETS family of transcription factors, however, has been implicated previously in development of erythroleukemia. The v-ets oncogene, contained in the E26 avian acute leukemia virus, contributes to development of a mixed myelo-erythroid leukemia in chickens and is capable of transforming erythroblasts in culture (21, 22). In addition, a closely related ETS family member, friend leukemia virus integration 1 (FLI1), is a common site of retroviral integration in Friend virus–induced erythroleukemias (23). Our results, thus, suggest that an investigation into the role ERG may play in human erythroleukemia may now be warranted.
Materials and Methods
Retroviral Constructs and Virus Production.
Murine Erg cDNA (GenBank accession no. NM_133659), with an N-terminal FLAG tag, was subcloned into an MSCV-IRES-mCherry retroviral backbone using EcoRI and XhoI restriction endonucleases. Viral supernatant was produced in phoenix-E cells as described (24). Viral supernatants were collected 48 h posttransfection and stored at −80 °C.
Immunoblotting.
Spleen cells from leukemic and control mice were lysed in RIPA buffer [1% (vol/vol) Triton X-100, 0.1% SDS, 1% sodium deoxycholate, 150 mM NaCl, 10 mM Tris-HCl (pH 7.5), 0.01% (wt/vol) sodium azide] on ice for 30 min, and proteins were separated using SDS/PAGE. The proteins were transferred to a PVDF membrane and blotted with a FLAG M2 monoclonal antibody to detect FLAG-tagged Erg.
Hematopoietic Progenitor Cell Transduction and Transplantation.
E12.5–E13.5 C57BL/6 fetal livers were dissociated in PBS supplemented with 10% (vol/vol) FCS and incubated with 4 μg/mL rat anti-Ter119 antibody. Ter119+ cells were removed using goat anti-rat IgG biomag beads (Qiagen) and a Dynal magnet. The remaining hematopoietic cells were then transferred to six-well plates coated with retronectin (Takara Bio), retroviral supernatant, and 6 μg/mL polybrene (Sigma). For 5-FU bone marrow cell transductions, C57BL/6 mice were given a single i.v. injection of 150 mg/kg 5-FU (Hospira), and bone marrow was harvested 4 d later. The harvested cells were transduced with retrovirus as above. Transduced cells were then incubated overnight at 37 °C in Dulbecco’s modified Eagle medium (DMEM) supplemented with 10% (vol/vol) FCS, SCF (100 ng/mL), IL-6 (10 ng/mL), Flt3L (5 ng/mL), and TPO (50 ng/mL). Cells were injected i.v. into the tail vein of lethally (2× 550 rad) or sublethally (1× 550 rad) irradiated C57BL/6.Ly5.1 congenic recipient mice. Between 300,000 and 500,000 cells were injected per recipient. For secondary transplantation analysis, 107 primary spleen or bone marrow cells were injected i.v. into unirradiated or sublethally irradiated (1× 550 rad) syngeneic C57BL/6 mice.
Histopathology and Clonogenic Cell Analysis.
Organs were harvested from moribund and control mice, weighed, and fixed in 10% (vol/vol) buffered formalin and paraffin-embedded. Sections (4 μm) were stained with hematoxylin and eosin for general cytological analysis or acetylcholinesterase for megakaryocyte analysis. Retroorbital blood was collected into EDTA-coated tubes, and differential counts were performed manually on May–Grunwald–Giemsa–stained blood smears or automatically using an ADVIA 120 blood analyzer equipped with a mouse analysis software module (Bayer). Clonal agar cultures were performed as described (25). Methylcellulose cultures were incubated for 7 or 14 d at 37 °C and 5% (vol/vol) CO2 in duplicate 1.1-mL, 35-mm dishes of Methylcult 3234 (Stem Cell Technologies) supplemented with 100 ng/mL SCF, 10 ng/mL IL-3, and 4 IU/mL EPO. Individual methylcellulose colonies were picked using a Pasteur pipette and dissociated into DMEM/10% (vol/vol) FCS. Resuspended cells were then stained for flow cytometric analysis or cytocentrifuged for cytological analysis. For cytological analysis, cells were cytocentrifuged onto slides. Slides were stained with May–Grunwald–Giemsa or, for acetylcholinesterase, followed by Luxol Fast Blue and hematoxylin. An unpaired one-tailed t test was performed on spleen and liver weights.
Flow Cytometry.
Bone marrow cells were harvested from two femurs into PBS/10% (vol/vol) FCS, and spleen and thymus cells were dissociated into PBS/10% (vol/vol) FCS using a 100-μm sieve. Cells were stained with the following antibodies: Ter119-APC, CD71-FITC, Mac1-PerCpCy5.5, Gr1-PeCY7, B220-APC, CD4-FITC, CD8-APC, CD41-PeCY7, and cKIT-PerCpCy5.5 (BD Biosciences); and fluorogold was used as a viability marker. Methylcellulose colony cells were stained with Ter119-APC, CD71-FITC, and CD41-PeCY7 (BD Biosciences). A LSRII (BD Biosciences) flow cytometer was used to measure cell surface staining.
Supplementary Material
Acknowledgments
We thank Emma Lanera and Lauren Wilkins for outstanding animal husbandry and Jason Corbin for automated peripheral blood analysis. This work was supported by Project Grant 516726, Program Grants 461219 and 1016647, and an Independent Research Institutes Infrastructure Support Scheme Grant 361646 from the Australian National Health and Medical Research Council (NHMRC); fellowships from the Leukaemia Foundation (to C.L.C.), the Sylvia and Charles Viertel Foundation (to B.T.K.), the NHMRC (to W.S.A. and B.T.K.), and the Cancer Council of Victoria (to D.M.); a University of Melbourne scholarship (to E.A.K.); the Australian Cancer Research Fund; and a Victorian State Government Operational Infrastructure Support grant.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1213454109/-/DCSupplemental.
References
- 1.Ng AP, et al. Erg is required for self-renewal of hematopoietic stem cells during stress hematopoiesis in mice. Blood. 2011;118:2454–2461. doi: 10.1182/blood-2011-03-344739. [DOI] [PubMed] [Google Scholar]
- 2.Taoudi S, et al. ERG dependence distinguishes developmental control of hematopoietic stem cell maintenance from hematopoietic specification. Genes Dev. 2011;25:251–262. doi: 10.1101/gad.2009211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Loughran SJ, et al. The transcription factor Erg is essential for definitive hematopoiesis and the function of adult hematopoietic stem cells. Nat Immunol. 2008;9:810–819. doi: 10.1038/ni.1617. [DOI] [PubMed] [Google Scholar]
- 4.Tomlins SA, et al. Recurrent fusion of TMPRSS2 and ETS transcription factor genes in prostate cancer. Science. 2005;310:644–648. doi: 10.1126/science.1117679. [DOI] [PubMed] [Google Scholar]
- 5.Tomlins SA, et al. Role of the TMPRSS2-ERG gene fusion in prostate cancer. Neoplasia. 2008;10:177–188. doi: 10.1593/neo.07822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Klezovitch O, et al. A causal role for ERG in neoplastic transformation of prostate epithelium. Proc Natl Acad Sci USA. 2008;105:2105–2110. doi: 10.1073/pnas.0711711105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ichikawa H, Shimizu K, Hayashi Y, Ohki M. An RNA-binding protein gene, TLS/FUS, is fused to ERG in human myeloid leukemia with t(16;21) chromosomal translocation. Cancer Res. 1994;54:2865–2868. [PubMed] [Google Scholar]
- 8.Sorensen PH, et al. A second Ewing’s sarcoma translocation, t(21;22), fuses the EWS gene to another ETS-family transcription factor, ERG. Nat Genet. 1994;6:146–151. doi: 10.1038/ng0294-146. [DOI] [PubMed] [Google Scholar]
- 9.Marcucci G, et al. Cancer and Leukemia Group B Study High expression levels of the ETS-related gene, ERG, predict adverse outcome and improve molecular risk-based classification of cytogenetically normal acute myeloid leukemia: A Cancer and Leukemia Group B Study. J Clin Oncol. 2007;25:3337–3343. doi: 10.1200/JCO.2007.10.8720. [DOI] [PubMed] [Google Scholar]
- 10.Baldus CD, et al. High expression of the ETS transcription factor ERG predicts adverse outcome in acute T-lymphoblastic leukemia in adults. J Clin Oncol. 2006;24:4714–4720. doi: 10.1200/JCO.2006.06.1580. [DOI] [PubMed] [Google Scholar]
- 11.Ng AP, et al. Trisomy of Erg is required for myeloproliferation in a mouse model of Down syndrome. Blood. 2010;115:3966–3969. doi: 10.1182/blood-2009-09-242107. [DOI] [PubMed] [Google Scholar]
- 12.Rainis L, et al. The proto-oncogene ERG in megakaryoblastic leukemias. Cancer Res. 2005;65:7596–7602. doi: 10.1158/0008-5472.CAN-05-0147. [DOI] [PubMed] [Google Scholar]
- 13.Roy A, Roberts I, Norton A, Vyas P. Acute megakaryoblastic leukaemia (AMKL) and transient myeloproliferative disorder (TMD) in Down syndrome: A multi-step model of myeloid leukaemogenesis. Br J Haematol. 2009;147:3–12. doi: 10.1111/j.1365-2141.2009.07789.x. [DOI] [PubMed] [Google Scholar]
- 14.Zipursky A. Transient leukaemia—a benign form of leukaemia in newborn infants with trisomy 21. Br J Haematol. 2003;120:930–938. doi: 10.1046/j.1365-2141.2003.04229.x. [DOI] [PubMed] [Google Scholar]
- 15.Stankiewicz MJ, Crispino JD. ETS2 and ERG promote megakaryopoiesis and synergize with alterations in GATA-1 to immortalize hematopoietic progenitor cells. Blood. 2009;113:3337–3347. doi: 10.1182/blood-2008-08-174813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kirsammer G, et al. Highly penetrant myeloproliferative disease in the Ts65Dn mouse model of Down syndrome. Blood. 2008;111:767–775. doi: 10.1182/blood-2007-04-085670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Malinge S, et al. Increased dosage of the chromosome 21 ortholog Dyrk1a promotes megakaryoblastic leukemia in a murine model of Down syndrome. J Clin Invest. 2012;122:948–962. doi: 10.1172/JCI60455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tsuzuki S, Taguchi O, Seto M. Promotion and maintenance of leukemia by ERG. Blood. 2011;117:3858–3868. doi: 10.1182/blood-2010-11-320515. [DOI] [PubMed] [Google Scholar]
- 19.Thoms JA, et al. ERG promotes T-acute lymphoblastic leukemia and is transcriptionally regulated in leukemic cells by a stem cell enhancer. Blood. 2011;117:7079–7089. doi: 10.1182/blood-2010-12-317990. [DOI] [PubMed] [Google Scholar]
- 20.Salek-Ardakani S, et al. ERG is a megakaryocytic oncogene. Cancer Res. 2009;69:4665–4673. doi: 10.1158/0008-5472.CAN-09-0075. [DOI] [PubMed] [Google Scholar]
- 21.Metz T, Graf T. v-myb and v-ets transform chicken erythroid cells and cooperate both in trans and in cis to induce distinct differentiation phenotypes. Genes Dev. 1991;5:369–380. doi: 10.1101/gad.5.3.369. [DOI] [PubMed] [Google Scholar]
- 22.Radke K, Beug H, Kornfeld S, Graf T. Transformation of both erythroid and myeloid cells by E26, an avian leukemia virus that contains the myb gene. Cell. 1982;31:643–653. doi: 10.1016/0092-8674(82)90320-8. [DOI] [PubMed] [Google Scholar]
- 23.Ben-David Y, Giddens EB, Letwin K, Bernstein A. Erythroleukemia induction by Friend murine leukemia virus: Insertional activation of a new member of the ets gene family, Fli-1, closely linked to c-ets-1. Genes Dev. 1991;5:908–918. doi: 10.1101/gad.5.6.908. [DOI] [PubMed] [Google Scholar]
- 24.Pear WS, et al. Efficient and rapid induction of a chronic myelogenous leukemia-like myeloproliferative disease in mice receiving P210 bcr/abl-transduced bone marrow. Blood. 1998;92:3780–3792. [PubMed] [Google Scholar]
- 25.Alexander WS, Roberts AW, Nicola NA, Li R, Metcalf D. Deficiencies in progenitor cells of multiple hematopoietic lineages and defective megakaryocytopoiesis in mice lacking the thrombopoietic receptor c-Mpl. Blood. 1996;87:2162–2170. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.