Abstract
The limit of the Colletotrichum gloeosporioides species complex is defined genetically, based on a strongly supported clade within the Colletotrichum ITS gene tree. All taxa accepted within this clade are morphologically more or less typical of the broadly defined C. gloeosporioides, as it has been applied in the literature for the past 50 years. We accept 22 species plus one subspecies within the C. gloeosporioides complex. These include C. asianum, C. cordylinicola, C. fructicola, C. gloeosporioides, C. horii, C. kahawae subsp. kahawae, C. musae, C. nupharicola, C. psidii, C. siamense, C. theobromicola, C. tropicale, and C. xanthorrhoeae, along with the taxa described here as new, C. aenigma, C. aeschynomenes, C. alatae, C. alienum, C. aotearoa, C. clidemiae, C. kahawae subsp. ciggaro, C. salsolae, and C. ti, plus the nom. nov. C. queenslandicum (for C. gloeosporioides var. minus). All of the taxa are defined genetically on the basis of multi-gene phylogenies. Brief morphological descriptions are provided for species where no modern description is available. Many of the species are unable to be reliably distinguished using ITS, the official barcoding gene for fungi. Particularly problematic are a set of species genetically close to C. musae and another set of species genetically close to C. kahawae, referred to here as the Musae clade and the Kahawae clade, respectively. Each clade contains several species that are phylogenetically well supported in multi-gene analyses, but within the clades branch lengths are short because of the small number of phylogenetically informative characters, and in a few cases individual gene trees are incongruent. Some single genes or combinations of genes, such as glyceraldehyde-3-phosphate dehydrogenase and glutamine synthetase, can be used to reliably distinguish most taxa and will need to be developed as secondary barcodes for species level identification, which is important because many of these fungi are of biosecurity significance. In addition to the accepted species, notes are provided for names where a possible close relationship with C. gloeosporioides sensu lato has been suggested in the recent literature, along with all subspecific taxa and formae speciales within C. gloeosporioides and its putative teleomorph Glomerella cingulata.
Taxonomic novelties:
Name replacement - C. queenslandicum B. Weir & P.R. Johnst. New species - C. aenigma B. Weir & P.R. Johnst., C. aeschynomenes B. Weir & P.R. Johnst., C. alatae B. Weir & P.R. Johnst., C. alienum B. Weir & P.R. Johnst, C. aotearoa B. Weir & P.R. Johnst., C. clidemiae B. Weir & P.R. Johnst., C. salsolae B. Weir & P.R. Johnst., C. ti B. Weir & P.R. Johnst. New subspecies - C. kahawae subsp. ciggaro B. Weir & P.R. Johnst. Typification: Epitypification - C. queenslandicum B. Weir & P.R. Johnst.
Key words: anthracnose, Ascomycota, barcoding, Colletotrichum gloeosporioides, Glomerella cingulata, phylogeny, systematics
INTRODUCTION
The name Colletotrichum gloeosporioides was first proposed in Penzig (1882), based on Vermicularia gloeosporioides, the type specimen of which was collected from Citrus in Italy. Much of the early literature used this name to refer to fungi associated with various diseases of Citrus, with other species established for morphologically similar fungi from other hosts. However, several early papers discussed the morphological similarity between many of the Colletotrichum spp. that had been described on the basis of host preference, and used inoculation tests to question whether or not the species were distinct. Some of these papers investigated in culture the link between the various Colletotrichum species and their sexual Glomerella state (e.g. Shear & Wood 1907, Ocfemia & Agati 1925). Authors such as Shear & Wood (1907, 1913) and Small (1926) concluded that many of the species described on the basis of host preference were in fact the same, rejecting apparent differences in host preference as a basis for taxonomic segregation. Small (1926) concluded that the names Glomerella cingulata and Colletotrichum gloeosporioides should be used for the sexual and asexual morphs, respectively, of the many Colletotrichum spp. they regarded as conspecific. Colletotrichum gloeosporioides was stated to be the earliest name with a proven link to what they regarded as a biologically diverse G. cingulata. The studies of von Arx & Müller (1954) and von Arx (1957, 1970) taxonomically formalised this concept.
The “von Arxian” taxonomic concept for Colletotrichum saw large numbers of species synonymised with the names C. graminicola (for grass-inhabiting species) and C. gloeosporioides (for non-grass inhabiting species with straight conidia). The genetic and biological diversity encompassed by these names was so broad that they became of little practical use to plant pathologists, conveying no information about pathogenicity, host range, or other attributes. The von Arx & Müller (1954) and von Arx (1957) studies were not based on direct examination of type material of all species and some of the synonymy proposed in these papers has subsequently been found to be incorrect. Examples include the segregation of C. acutatum (Simmonds 1965) and C. boninense (Moriwaki et al. 2003) from C. gloeosporioides sensu von Arx (1957). Other studies published elsewhere in this volume (Damm et al. 2012a, b) show that several species regarded as synonyms of C. gloeosporioides by von Arx (1957) are members of the C. acutatum complex (e.g. C. godetiae, Gloeosporium limetticola, G. lycopersici, and G. phormii) or the C. boninense complex (e.g. C. dracaenae). Recent molecular studies have resulted in a much better understanding of phylogenetic relationships amongst the grass-inhabiting species of the C. graminicola group and the development of a more useful taxonomy for this group of fungi (e.g. Hsiang & Goodwin 2001, Du et al. 2005, and Crouch et al. 2006). This group is now recognised as comprising several host-specialised, genetically well characterised species, but a modern taxonomy for C. gloeosporioides has yet to be resolved.
Von Arx (1970) and Sutton (1980) distinguished the C. gloeosporioides group using conidial shape and size. A few apparently host-specialised, C. gloeosporioides-like taxa were retained by these authors, but the basis of their identification was often difficult to understand. Prior to the availability of DNA sequence data, taxonomic concepts within Colletotrichum were based on features such as host species, substrate, conidial size and shape, shape of appressoria, growth rate in culture, colour of cultures, presence or absence of setae, whether or not the teleomorph develops, etc. Some studies have found characters such as these useful for distinguishing groups within C. gloeosporioides (e.g. Higgins 1926, Gorter 1956, Hindorf 1973, and Johnston & Jones 1997). However, problems arise because many of these morphological features change under different conditions of growth (dependent upon growth media, temperature, light regime, etc.), or can be lost or change with repeated subculturing. Host preference is poorly controlled — even good, well-defined pathogens causing a specific disease can be isolated by chance from other substrates (e.g. Johnston 2000). Colletotrichum conidia will germinate on most surfaces, form an appressorium, remain attached to that surface as a viable propagule or perhaps as a minor, endophytic or latent infection, and grow out from there into senescing plant tissue or onto agar plates if given the opportunity. In addition, the same disease can be caused by genetically distinct sets of isolates, the shared pathogenicity presumably independently evolved, e.g. the bitter rot disease of apple is caused by members of both the C. acutatum and C. gloeosporioides species complexes (Johnston et al. 2005).
Sutton (1992) commented on C. gloeosporioides that “No progress in the systematics and identification of isolates belonging to this complex is likely to be made based on morphology alone”. A start was made towards a modern understanding of this name with the designation of an epitype specimen with a culture derived from it to stabilise the application of the name (Cannon et al. 2008). Based on ITS sequences, the ex-epitype isolate belongs in a strongly supported clade, distinct from other taxa that have been confused with C. gloeosporioides in the past, such as C. acutatum and C. boninense (e.g. Abang et al. 2002, Martinez-Culebras et al. 2003, Johnston et al. 2005, Chung et al. 2006, Farr et al. 2006, Than et al. 2008). However, biological and genetic relationships within the broad C. gloeosporioides clade remain confused and ITS sequences alone are insufficient to resolve them.
In this study we define the limits of the C. gloeosporioides species complex on the basis of ITS sequences, the species we accept within the complex forming a strongly supported clade in the ITS gene tree (fig. 1 in Cannon et al. 2012, this issue). In all cases the taxa we include in the C. gloeosporioides complex would fit within the traditional morphological concept of the C. gloeosporioides group (e.g. von Arx 1970, Mordue 1971, and Sutton 1980). Commonly used species names within the C. gloeosporioides complex include C. fragariae, C. musae, and C. kahawae. Since the epitype paper (Cannon et al. 2008), several new C. gloeosporioides-like species have been described in regional studies, where multi-gene analyses have shown the new species to be phylogenetically distinct from the ex-epitype strain of C. gloeosporioides (e.g. Rojas et al. 2010, Phoulivong et al. 2011, and Wikee et al. 2011).
The regional nature of most of these studies, the often restricted genetic sampling across the diversity of C. gloeosporioides globally, and the minimal overlap between isolates treated and gene regions targeted in the various studies, means that the relationship between the newly described species is often poorly understood.
While some authors have embraced a genetically highly restricted concept for C. gloeosporioides, many applied researchers continue to use the name in a broad, group-species concept (e.g. Bogo et al. 2012, Deng et al. 2012, Kenny et al. 2012, Parvin et al. 2012, and Zhang et al. 2012). In this paper we accept both concepts as useful and valid. When used in a broad sense, we refer to the taxon as the C. gloeosporioides species complex or C. gloeosporioides s. lat.
This paper aims to clarify the genetic and taxonomic relationships within the C. gloeosporioides species complex using a set of isolates that widely samples its genetic, biological and geographic diversity. Type specimens, or cultures derived from type specimens, have been examined wherever possible. Although we do not treat all of the names placed in synonymy with C. gloeosporioides or Glomerella cingulata by von Arx & Müller (1954) and von Arx (1957, 1970), we treat all names for which a possible close relationship with C. gloeosporioides has been suggested in the recent literature, along with all subspecific taxa and formae speciales within C. gloeosporioides and G. cingulata.
ITS sequences, the official barcoding gene for fungi (Seifert 2009, Schoch et al. 2012), do not reliably resolve relationships within the C. gloeosporioides complex. We define species in the complex genetically rather than morphologically, on the basis of phylogenetic analyses of up to eight genes. Following Cannon et al. (2012, this issue) the generic name Colletotrichum is used as the preferred generic name for all species wherever possible throughout this paper, whether or not a Glomerella state has been observed for that fungus, and whether or not the Glomerella state has a formal name.
MATERIALS AND METHODS
Specimen isolation and selection
An attempt was made to sample the genetic diversity across C. gloeosporioides as widely as possible, with isolates from diverse hosts from around the world selected for more intensive study. A BLAST search of GenBank using the ITS sequence of the epitype culture of C. gloeosporioides (Cannon et al. 2008) provided a coarse estimate for the genetic limit of the C. gloeosporioides complex and ITS diversity across the complex was used to select a genetically diverse set of isolates. Voucher cultures were obtained from the research groups who deposited the GenBank records. To these were added isolates representing the known genetic and morphological diversity of C. gloeosporioides from New Zealand, isolated from rots of native and introduced fruits, from diseased exotic weeds, and as endophytes from leaves of native podocarps. Additional isolates representing ex-type and authentic cultures of as many named taxa and formae speciales within the C. gloeosporioides complex as possible were obtained from international culture collections. Approximately 400 isolates belonging to the C. gloeosporioides complex were obtained. GAPDH gene sequences were generated for all isolates as an initial measure of genetic diversity. A subset of 156 isolates, selected to represent the range of genetic, geographic, and host plant diversity, was used in this research (Table 1).
Table 1.
A list of strains used in this study.
= ex-type or authentic culture, (*) = ex-type or authentic culture of synonymised taxon. Sequences downloaded from GenBank, not generated as part of this project are in bold font. Collection abbreviations are listed in the methods.
Most of the New Zealand isolates had been stored as conidial suspensions made from single conidium or ascospore cultures and then stored at -80 °C in a 5 % glycerol/water suspension. Additional isolates from New Zealand were obtained from the ICMP culture collection, where isolates are stored as lyophilised (freeze-dried) ampoules or in a metabolically inactive state in liquid nitrogen at -196 °C. The storage history of most of the isolates received from other research groups is not known. Table 1 lists the isolates studied. All those supplying cultures are acknowledged at the end of this manuscript, and additional details on each culture are available on the ICMP website (http://www.landcareresearch.co.nz/resources/collections/icmp).
Culture collection and fungal herbarium (fungarium) abbreviations used herein are: CBS = Centraalbureau voor Schimmelcultures (Netherlands), ICMP = International Collection of Microorganisms from Plants, MFLU = Mae Fah Luang University Herbarium (Thailand) MFLUCC = Mae Fah Luang University Culture Collection (Thailand), GCREC = University of Florida, Gulf Coast Research and Education Centre (USA), HKUCC = The University of Hong Kong Culture Collection (China), IMI = CABI Genetic Resource Collection (UK), MAFF = Ministry of Agriculture, Forestry and Fisheries (Japan), DAR = Plant Pathology Herbarium (Australia), NBRC = Biological Resource Center, National Institute of Technology and Evaluation (Japan), BCC = BIOTEC Culture Collection (Thailand), GZAAS = Guizhou Academy of Agricultural Sciences herbarium (China), MUCL = Belgian Co-ordinated Collections of Micro-organisms, (agro)industrial fungi & yeasts (Belgium), BRIP = Queensland Plant Pathology Herbarium (Australia), PDD = New Zealand Fungal and Plant Disease Collection (New Zealand), BPI = U.S. National Fungus Collections (USA), STE-U = Culture collection of the Department of Plant Pathology, University of Stellenbosch (South Africa), and MCA = M. Catherine Aime’s collection series, Louisiana State University (USA).
DNA extraction, amplification, and sequencing
Mycelium was collected from isolates grown on PDA agar, and manually comminuted with a micropestle in 420 μL of Quiagen DXT tissue digest buffer; 4.2 μL of proteinase K was added and incubated at 55 °C for 1 h. After a brief centrifugation 220 μL of the supernatant was placed in a Corbett X-tractorGene automated nucleic acid extraction robot. The resulting 100 μL of pure DNA in TE buffer was stored at -30 °C in 1.5 mL tubes until use.
Gene sequences were obtained from eight nuclear gene regions, actin (ACT) [316 bp], calmodulin (CAL) [756 bp], chitin synthase (CHS-1) [229 bp], glyceraldehyde-3-phosphate dehydrogenase (GAPDH) [308 bp], the ribosomal internal transcribed spacer (ITS) [615 bp], glutamine synthetase (GS) [907 bp], manganese-superoxide dismutase (SOD2) [376 bp], and β-tubulin 2 (TUB2) [716 bp].
PCR Primers used during this study are shown in Table 2. The standard CAL primers (O’Donnell et al. 2000) gave poor or non-specific amplification for most isolates, thus new primers (CL1C, CL2C) were designed for Colletotrichum based on the C. graminicola M1.001 genome sequence. The standard GS primers (Stephenson et al. 1997) sequenced poorly for some isolates due to an approx. 9 bp homopolymer T run 71 bp in from the end of the GSF1 primer binding site. A new primer, GSF3, was designed 41 bp downstream of this region to eliminate the homopolymer slippage error from sequencing. The reverse primer GSR2 was designed in the same location as GSR1 with one nucleotide change. Both new GS primers were based on similarity with a C. theobromicola UQ62 sequence (GenBank L78067, as C. gloeosporioides).
Table 2.
Primers used in this study, with sequences and sources.
| Gene | Product name | Primer | Direction | Sequence (5’–3’) | Reference |
|---|---|---|---|---|---|
| ACT | Actin | ACT-512F | Foward | ATG TGC AAG GCC GGT TTC GC | Carbone & Kohn 1999 |
| ACT-783R | Reverse | TAC GAG TCC TTC TGG CCC AT | Carbone & Kohn 1999 | ||
| CAL | Calmodulin | CL1 | Foward | GAR TWC AAG GAG GCC TTC TC | O’Donnell et al. 2000 |
| CL2A | Reverse | TTT TTG CAT CAT GAG TTG GAC | O’Donnell et al. 2000 | ||
| CL1C | Foward | GAA TTC AAG GAG GCC TTC TC | This study | ||
| CL2C | Reverse | CTT CTG CAT CAT GAG CTG GAC | This study | ||
| CHS-1 | Chitin synthase | CHS-79F | Foward | TGG GGC AAG GAT GCT TGG AAG AAG | Carbone & Kohn 1999 |
| CHS-345R | Reverse | TGG AAG AAC CAT CTG TGA GAG TTG | Carbone & Kohn 1999 | ||
| GAPDH | Glyceraldehyde-3-phosphate dehydrogenase | GDF | Foward | GCC GTC AAC GAC CCC TTC ATT GA | Templeton et al. 1992 |
| GDR | Reverse | GGG TGG AGT CGT ACT TGA GCA TGT | Templeton et al. 1992 | ||
| GS | Glutamine synthetase | GSF1 | Foward | ATG GCC GAG TAC ATC TGG | Stephenson et al. 1997 |
| GSF3 | Foward | GCC GGT GGA GGA ACC GTC G | This study | ||
| GSR1 | Reverse | GAA CCG TCG AAG TTC CAG | Stephenson et al. 1997 | ||
| GSR2 | Reverse | GAA CCG TCG AAG TTC CAC | This study | ||
| ITS | Internal transcribed spacer | ITS-1F | Foward | CTT GGT CAT TTA GAG GAA GTA A | Gardes & Bruns 1993 |
| ITS-4 | Reverse | TCC TCC GCT TAT TGA TAT GC | White et al. 1990 | ||
| SOD2 | Manganese-superoxide dismutase | SODglo2-F | Foward | CAG ATC ATG GAG CTG CAC CA | Moriwaki & Tsukiboshi 2009 |
| SODglo2-R | Reverse | TAG TAC GCG TGC TCG GAC AT | Moriwaki & Tsukiboshi 2009 | ||
| TUB2 | β-Tubulin 2 | T1 | Foward | AAC ATG CGT GAG ATT GTA AGT | O’Donnell & Cigelnik 1997 |
| T2 | Reverse | TAG TGA CCC TTG GCC CAGT TG | O’Donnell & Cigelnik 1997 | ||
| Bt2b | Reverse | ACC CTC AGT GTA GTG ACC CTT GGC | Glass & Donaldson 1995 |
The PCRs were performed in an Applied Biosystems Veriti Thermal Cycler in a total volume of 25 μL. The PCR mixtures contained 15.8 μL of UV-sterilised ultra-filtered water, 2.5 μL of 10× PCR buffer (with 20 mM MgCl2), 2.5 μL of dNTPs (each 20 μM), 1 μL of each primer (10 μM), 1 μL of BSA, 1 μL of genomic DNA, and 0.2 μL (1 U) of Roche FastStart Taq DNA Polymerase.
The PCR conditions for ITS were 4 min at 95 °C, then 35 cycles of 95 °C for 30 s, 52 °C for 30 s, 72 °C for 45 s, and then 7 min at 72 °C. The annealing temperatures differed for the other genes, with the optimum for each; ACT: 58 °C, CAL: 59 °C, CHS-1: 58 °C, GAPDH: 60 °C, GS: 54 °C, SOD2: 54 °C, TUB2: 55 °C. Some isolates required altered temperatures and occasionally gave multiple bands, which were excised separately from an electrophoresis gel and purified. PCR Products were purified on a Qiagen MinElute 96 UF PCR Purification Plate.
DNA sequences were obtained in both directions on an Applied Biosystems 3130xl Avant Genetic analyzer using BigDye v. 3.1 chemistry, electropherograms were analysed and assembled in Sequencher v. 4.10.1 (Gene Codes Corp.).
Phylogenetic analyses
Multiple sequence alignments of each gene were made with ClustalX v. 2.1 (Larkin et al. 2007), and manually adjusted where necessary with Geneious Pro v. 5.5.6 (Drummond et al. 2011).
Bayesian inference (BI) was used to reconstruct most of the phylogenies using MrBayes v. 3.2.1 (Ronquist et al. 2012). Bayesian inference has significant advantages over other methods of analysis such as maximum likelihood and maximum parsimony (Archibald et al. 2003) and provides measures of clade support as posterior probabilities rather than random resampling bootstraps. jModelTest v. 0.1.1 (Posada 2008) was used to carry out statistical selection of best-fit models of nucleotide substitution using the corrected Akaike information criteria (AICc) (Table 3). Initial analyses showed that individual genes were broadly congruent, thus nucleotide alignments of all genes were concatenated using Geneious, and separate partitions created for each gene with their own model of nucleotide substitution. Analyses on the full data set were run twice for 5 x 107 generations, and twice for 2 x 107 generations for the clade trees. Samples were taken from the posterior every 1000 generations. Convergence of all parameters was checked using the internal diagnostics of the standard deviation of split frequencies and performance scale reduction factors (PSRF), and then externally with Tracer v. 1.5 (Rambaut & Drummond 2007). On this basis the first 25 % of generations were discarded as burn-in.
Table 3.
Nucleotide substitution models used in phylogenetic analyses.
| Gene | All taxa | Musae clade | Kahawae clade |
|---|---|---|---|
| ITS | TrNef+G | TrNef+G | TrNef |
| GAPDH | HKY+G | TPM1uf+G | TrN |
| CAL | TIM1+G | TIM1+G | TrN+G |
| ACT | HKY+G | TrN | JC |
| CHS-1 | TrNef+G | TrNef+G | K80 |
| GS | TIM2+G | TIM3+G | |
| SOD2 | HKY+G | GTR+I+G | |
| TUB2 | TrN+G | HKY+G |
An initial BI analysis treated all 158 isolates using a concatenated alignment for five of the genes, ACT, CAL, CHS-1, GAPDH, and ITS. Colletotrichum boninense and C. hippeastri were used as outgroups. A second BI analysis, restricted to ex-type or authentic isolates of each of the accepted species, was based on a concatenated alignment of all eight genes. A third set of BI analyses treated focussed on taxa within the Musae clade and the Kahawae clade. For each clade, the ex-type or authentic isolates, together with 2–3 additional selected isolates of each accepted taxon where available, were analysed using a concatenated alignment of all eight genes, with C. gloeosporioides used as the outgroup for both analyses.
Several species-trees analyses were conducted using BEAST v. 1.7.1 (Drummond et al. 2012). Species-trees combine multi-gene and multiple isolate data to reconstruct the evolutionary history of hypothesised species, rather than individual isolates. BEAST does not use concatenation, but rather co-estimates the individual gene trees embedded inside the summary species tree. It also estimates the time since each species shared a common ancestor (divergence times). For these analyses the species tree ancestral reconstruction option was selected (Heled & Drummond 2010), the gene data partitioned as for BI and the substitution model for each gene was selected based on the models selected using jModelTest. The individual isolates were grouped into sets of species by setting species names as trait values. A strict clock was used for the GAPDH gene (as an all intronic sequence it was assumed to be accumulating mutations at a steady rate) and the other gene clock rates were estimated relative to GAPDH, using an uncorrelated lognormal relaxed clock. The species tree prior used for all genes was the Yule process, with the ploidy type set to nuclear autosomal. Uninformative priors were used for all parameters, and were allowed to auto optimise.
The first species-tree analysis was conducted using the 158 isolate, five gene dataset, with C. boninense and C. hippeastri as the outgroups. The MCMC chain was set to 1 × 108 generations for the species complex tree and samples were taken from the posterior every 1000 generations. The analysis was run twice independently. The effective sample size (ESS) and traces of all parameters and convergence of the two runs was checked with Tracer and a summary maximum clade credibility species tree was built with TreeAnnotator v. 1.7.1 (Drummond et al. 2012) using a 10 % burn-in and a posterior probability limit of 0.5, setting the heights of each node in the tree to the mean height across the entire sample of trees for that clade. Separate analyses were conducted using all eight genes and the same restricted set of isolates chosen to represent taxa within the Musae clade and the Kahawae clade as were used for the BI analyses of the eight gene concatenated analyses outlined above. For each of the Musae and Kahawae clade analyses, the MCMC chain was set to 5 × 107 generations, but otherwise run as for the five gene dataset.
To illustrate the potential limitations of ITS to discriminate species within the C. gloeosporioides complex, an UPGMA tree was built of all 158 ITS sequences, using the Geneious tree builder tool. A UPGMA tree visually approximates a BLAST search, which is based on distances (and sequence length) rather than corrected nucleotide substitutions of more sophisticated, model-based analyses.
Sequences derived in this study were lodged in GenBank (Table 1), the concatenated alignment and trees in TreeBASE (www.treebase.org) study number S12535, and taxonomic novelties in MycoBank (Crous et al. 2004).
Morphology
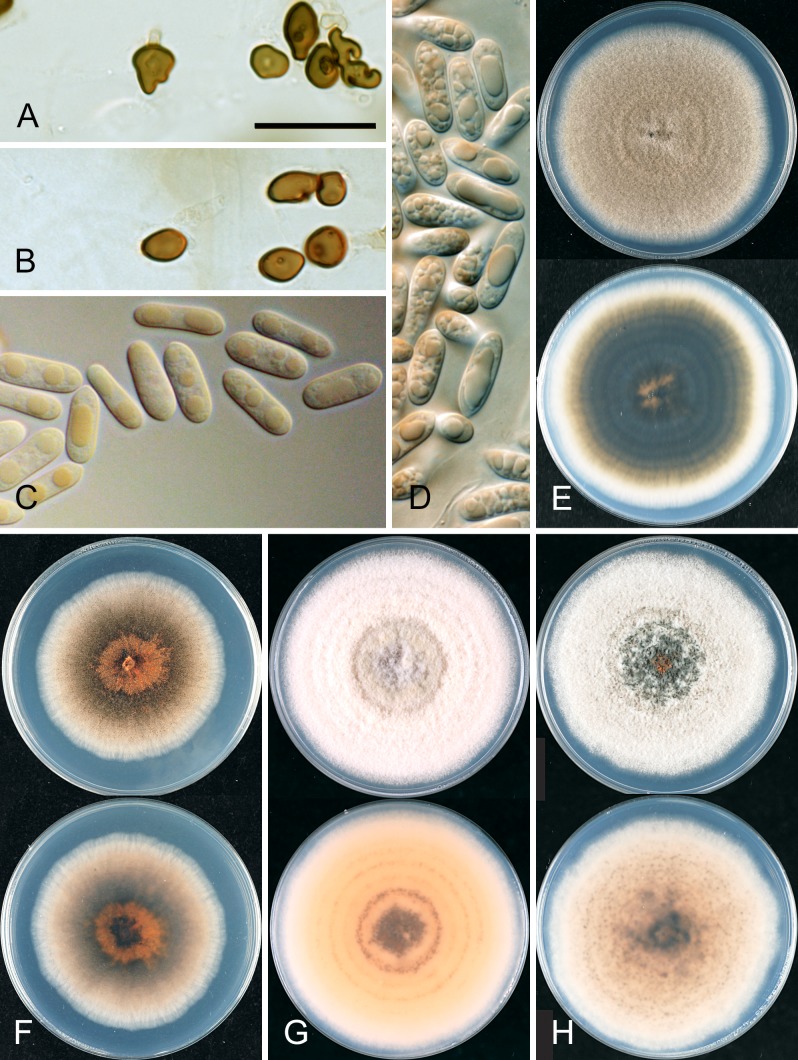
Detailed morphological descriptions are provided only for those species with no recently published description. Few specimens were examined from infected host material; the descriptions provided are mostly from agar cultures. Cultures were grown on Difco PDA from single conidia, or from single hyphal tips for the few specimens where no conidia were formed, with culture diameter measured and appearance described after 10 d growth at 18–20 °C under mixed white and UV fluorescent tubes, 12 h light/12 h dark. Colour codes follow Kornerup & Wanscher (1963).
Conidia were measured and described using conidia taken from the conidial ooze on acervuli and mounted in lactic acid, at least 24 conidia were measured for each isolate, range measurements are provided in the form (lower extreme–) 25 % quartile – 75 % quartile (–upper extreme), all ranges were rounded to the nearest 0.5 μm. Cultures were examined periodically for the development of perithecia. Ascospores were measured and described from perithecia crushed in lactic acid.
Appressoria were producing using a slide culture technique. A small square of agar was inoculated on one side with conidia and immediately covered with a sterile cover slip. After 14 d the cover slip was removed and placed in a drop of lactic acid on a glass slide.
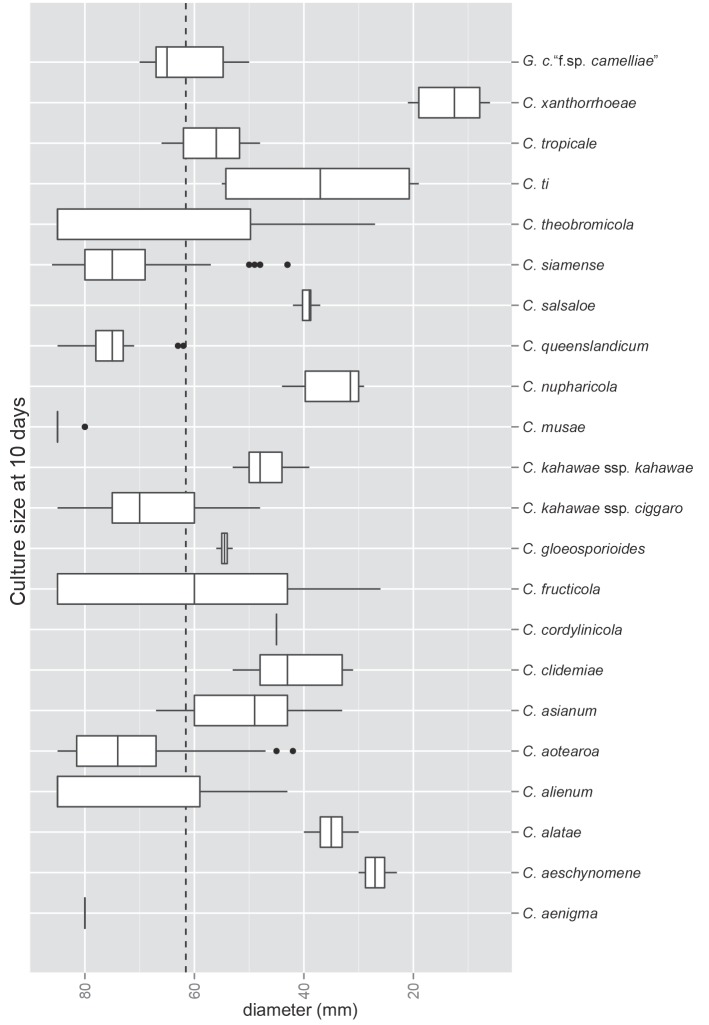
All morphological character measurements were analysed with the statistical programme “R” v. 2.14.0 (R Development Core Team 2011). The R package ggplot2 (Wickham 2009) was used for graphical plots. The box plots show the median, upper and lower quartiles, and the ‘whisker’ extends to the outlying data, or to a maximum of 1.5× the interquartile range, individual outliers outside this range are shown as dots.
Taxa treated in the taxonomic section
Species, subspecific taxa, and formae speciales within the C. gloeosporioides species complex are treated alphabetically by epithet. The names of formae speciales are not governed by the International Code of Botanical Nomenclature (ICBN) (McNeill et al. 2006, Art. 4, Note 4), and are hence enclosed in quotation marks to indicate their invalid status. Other invalid names that are governed by the ICBN are also enclosed in quotation marks. Accepted names are marked with an asterisk (*). The breadth of the taxonomic names treated includes:
all taxonomic names with DNA sequence data in GenBank that place them in the C. gloeosporioides complex as it has been defined here on the basis of the ITS gene tree. The sense that the names were used in GenBank may have been misapplied;
names that have been used in the literature in recent years for which a possible relationship to C. gloeosporioides has been suggested;
all subspecific taxa and formae speciales within C. gloeosporioides and Glomerella cingulata.
We have not considered the full set of species in Colletotrichum, Gloeosporium and Glomerella that were placed in synonymy with C. gloeosporioides or Glomerella cingulata by von Arx & Müller (1954) or von Arx (1957, 1970).
For each accepted species, comments are provided regarding the limitations of ITS, the official barcoding gene for fungi, to distinguish that species from others within the C. gloeosporioides complex.
RESULTS
Phylogenetics
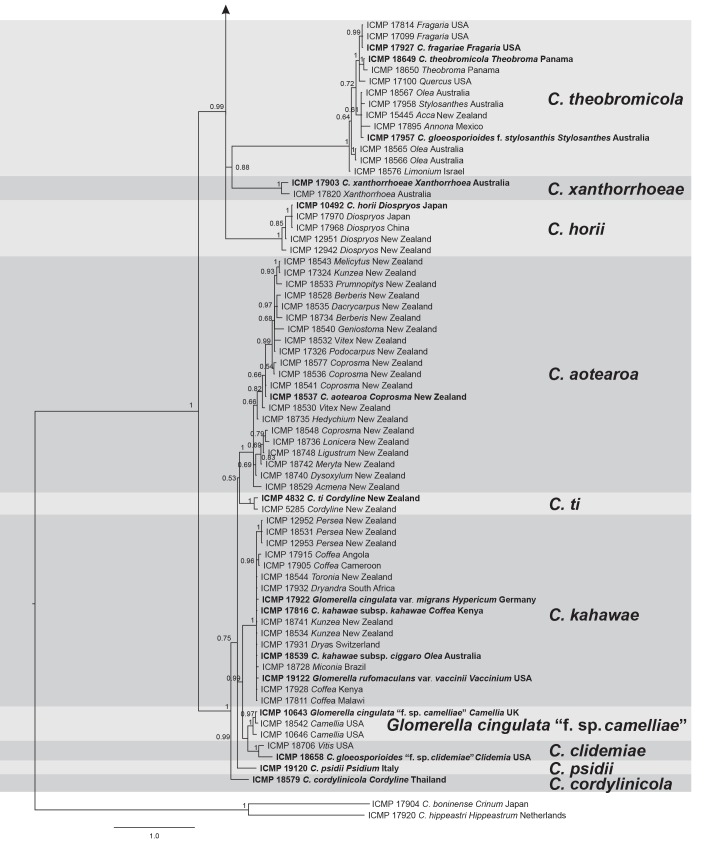
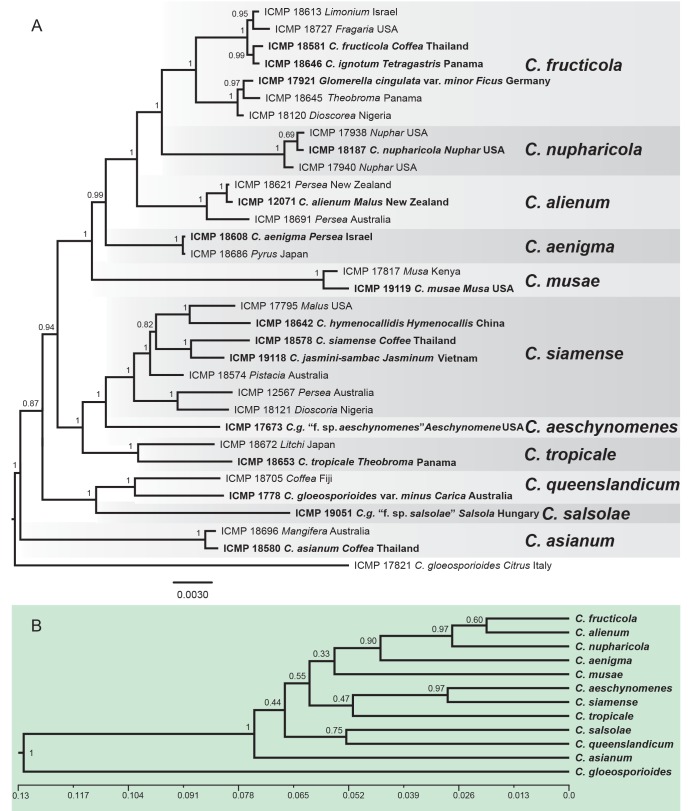
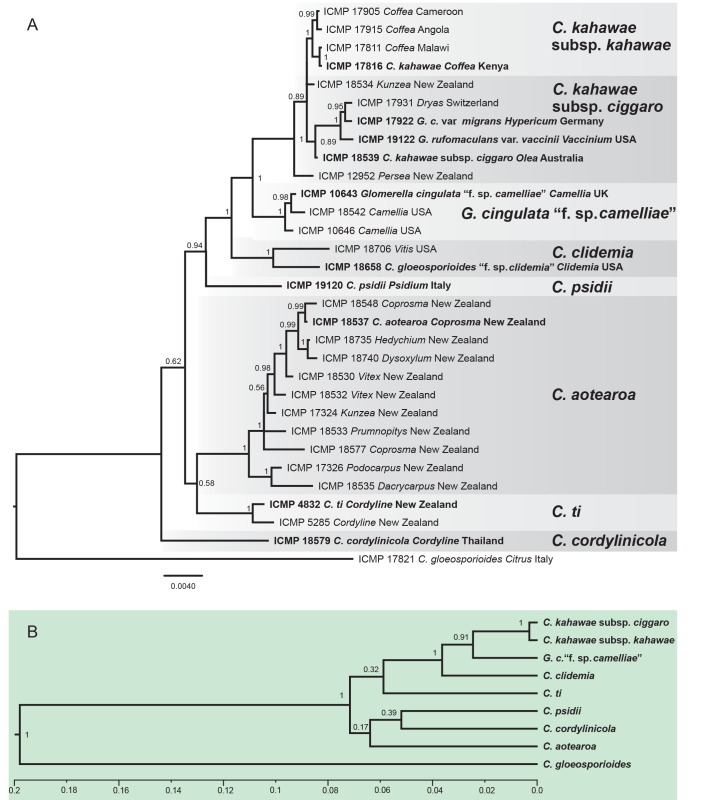
DNA sequences of five genes were obtained from all 158 isolates included in the study and concatenated to form a supermatrix of 2294 bp. The gene boundaries in the alignment were: ACT: 1–316, CAL: 317–1072, CHS-1: 1073–1371, GAPDH: 1372–1679, ITS: 1680–2294. A BI analysis of the concatenated dataset is presented in Fig.1. This tree is annotated with the species boundaries of the taxa that we accept in the C. gloeosporioides complex, and the clades representing these taxa formed the basis for investigating the morphological and biological diversity of our species. Ex-type and authentic isolates are highlighted in bold and labelled with the names under which they were originally described. The posterior probability (PP) support for the grouping of most species ranges from 1 to 0.96, however support for deeper nodes is often lower, e.g. 0.53 for the root of C. ti and C. aotearoa, indicating that the branching may be uncertain for the root of these species. Branch lengths and node PP are typically lower within a species than between species.
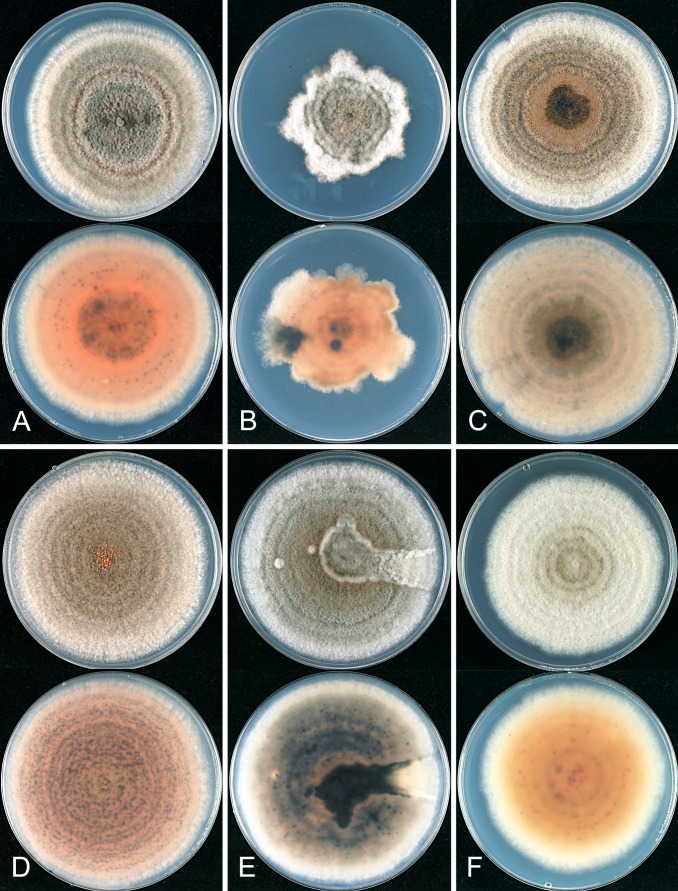
Fig. 1.
A Bayesian inference phylogenetic tree of 156 isolates in the Colletotrichum gloeosporioides species complex. The tree was built using concatenated sequences of the ACT, CAL, CHS-1, GAPDH, and ITS genes each with a separate model of DNA evolution. Bayesian posterior probability values ≥ 0.5 are shown above nodes. Culture accession numbers are listed along with host plant genus and country of origin. Ex-type and authentic cultures are emphasised in bold font, and include the taxonomic name as originally described. Species delimitations are indicated with grey boxes. Colletotrichum boninense and C. hippeastri isolates are used as outgroups. The scale bar indicates the number of expected changes per site.
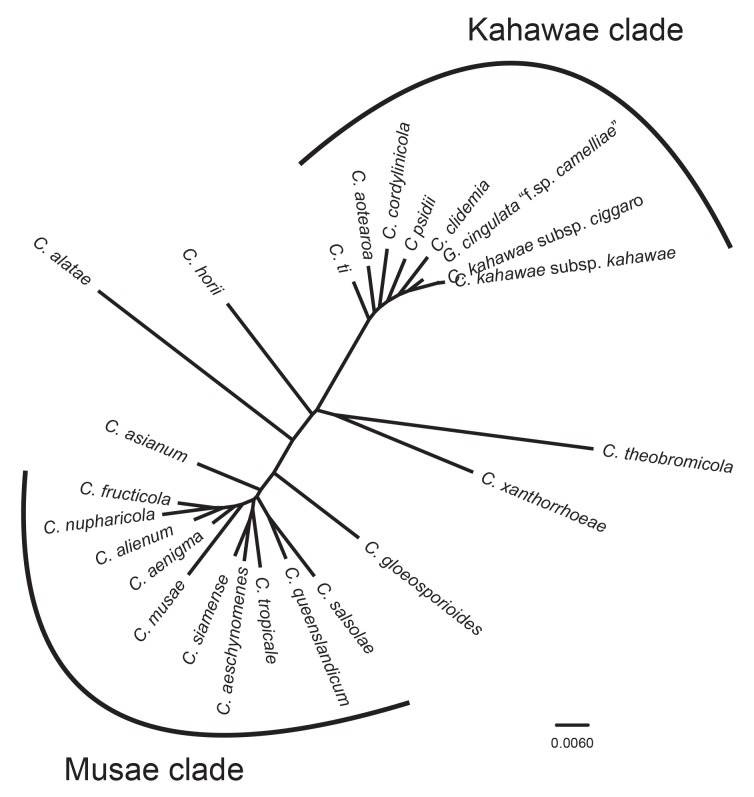
The large number of taxa in Fig. 1 makes it difficult to visualise the interspecific genetic distance between the recognised species. The unrooted tree in Fig. 2 represents the results of a BI analysis based on a concatenation of all eight genes, but restricted to the ex-type or authentic cultures from each of the accepted taxa. The analysis was done without out-group taxa and clearly shows two clusters of closely related species that we informally label the Musae clade, and the Kahawae clade.
Fig. 2.
An unrooted Bayesian inference phylogenetic tree of ex-type and authentic cultures of the 24 taxa within the Colletotrichum gloeosporioides species complex, illustrating their relative genetic distances, as indicated by branch lengths. There are two clusters within the species complex, the ‘Musae clade’ and the ‘Kahawae clade’. The tree was build using concatenated sequences of the ACT, TUB2, CAL, CHS-1, GAPDH, GS, ITS, and SOD2 genes each with a separate model of DNA evolution.
To better resolve relationships within the Musae and Kahawae clades a further set of BI analyses included eight genes and, wherever possible, several representative isolates of each of the accepted species. All eight gene sequences were concatenated to form a supermatrix for each clade. For the Musae clade of 32 isolates the alignment was 4199 bp and the gene boundaries were: ACT: 1–292, TUB2: 293–1008, CAL: 1009–1746, CHS-1: 1747–2045, GAPDH: 2046–2331, GS: 2332–3238, ITS: 3239–3823, SOD2: 3824–4199. For the Kahawae clade of 30 isolates the alignment was 4107 bp and the gene boundaries were: ACT: 1–281, TUB2: 282–988, CAL: 989–1728, CHS-1: 1729–2027, GAPDH: 2028–2311, GS: 2312–3179, ITS: 3198–3733, SOD2: 3734–4107. The additional genes sequenced provided additional support for our initial species delimitations with better resolution for some closely related species. For example, the highly pathogenic coffee berry isolates (referred to here as C. kahawae subsp. kahawae) were distinguished from other C. kahawae isolates.
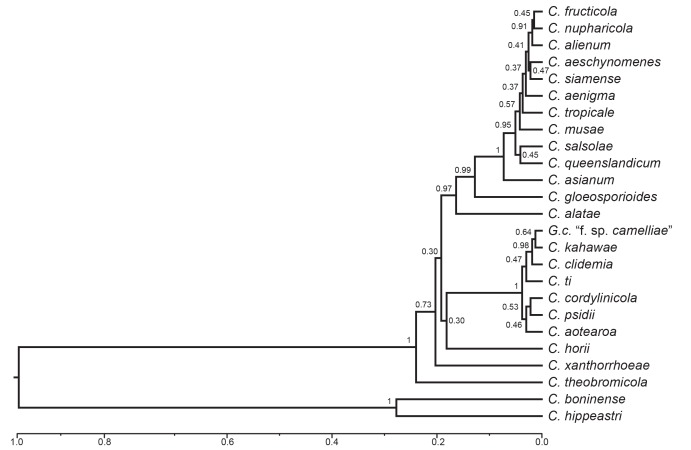
Analyses based on concatenated data sets can mask incongruence between individual gene trees. The low levels of support within some parts of the species-tree analysis (Fig. 3), in part reflects incongruence between gene trees. The levels of support for the Kahawae clade and for the Musae clade are strong (PP=1) but the species that we accept within these clades have lower levels of support than is shown between the other species outside of the clades. The scale bar in Fig. 3 represents a time scale, calibrated at zero for the present day, and at 1 for the last common ancestor (LCA) of the C. gloeosporioides and C. boninense species complexes. The separate species-tree analyses for the Musae and Kahawae clades provide a finer resolution of evolutionary history within each clade, the time scale based on the same calibration as Fig. 3.
Fig. 3.
A Bayesian inference species-tree of the C. gloeosporioides species complex. The tree was built by grouping all 158 isolates into species and simultaneously estimating the individual five gene trees (ACT, CAL, CHS-1, GAPDH, and ITS) and the summary species tree using BEAST. The scale is an uncalibrated clock set relative to the last common ancestor of the C. gloeosporioides and C. boninense species complexes.
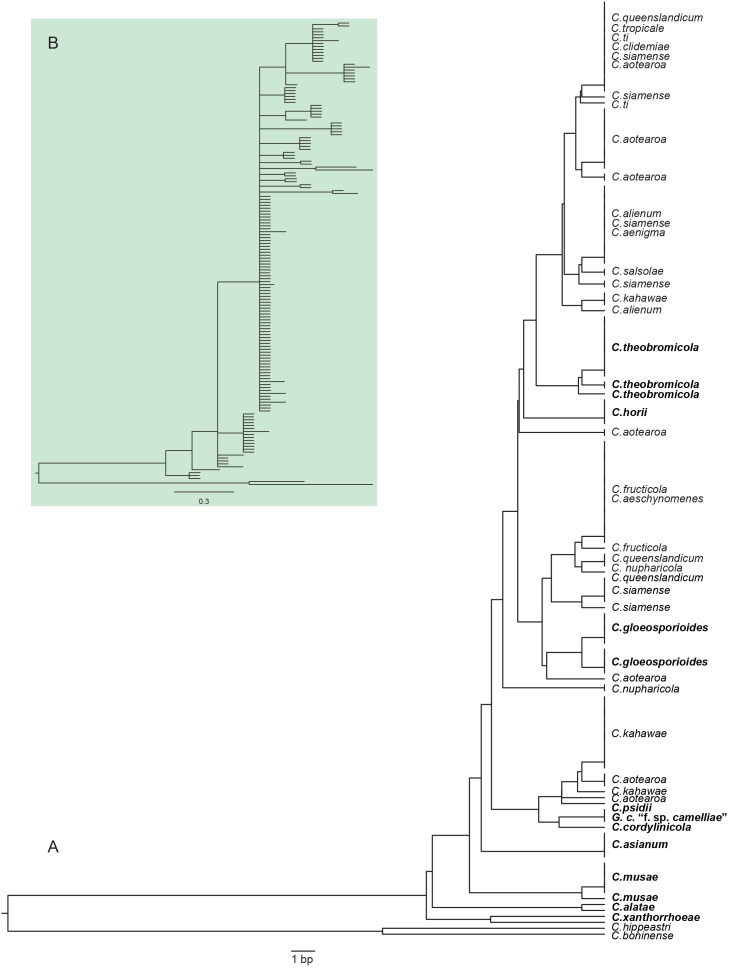
The UPGMA-based ITS gene tree (Fig 6). shows that C. theobromicola, C. horii, C. gloeosporioides, G. cingulata “f. sp.camelliae”, C. asianum, C. musae, C. alatae, C. xanthorrhoeae all form monophyletic clades and may be distinguished with ITS, but many species are unable to be discriminated using this gene alone. Note that C. cordylinicola and C. psidii are represented by a single isolate, meaning that variation within ITS sequences across these species has not been tested.
Fig. 6.
An UPGMA tree of ITS sequences from 156 isolates in the Colletotrichum gloeosporioides species complex. Isolate names have been replaced with species present in each clade. Species that are in monophyletic clades are emphasised in bold font to indicate those for which ITS barcoding is likely to work well. B: A 50 % majority-rule consensus Bayesian inference tree of the same data, showing the collapse of structure when analysed with a more robust method.
Morphology and biology
Brief morphological descriptions, based on all specimens examined, are provided for only those species with no recently published description. Conidial sizes for all accepted species are summarised in Fig. 7. Within a species, conidial sizes are reasonably consistent across isolates, independent of geographic origin or host. However, differences between species are often slight and size ranges often overlap (Fig. 7). The shape of appressoria is generally consistent within a species, some being simple in outline, others complex and highly lobed.
Fig. 7.
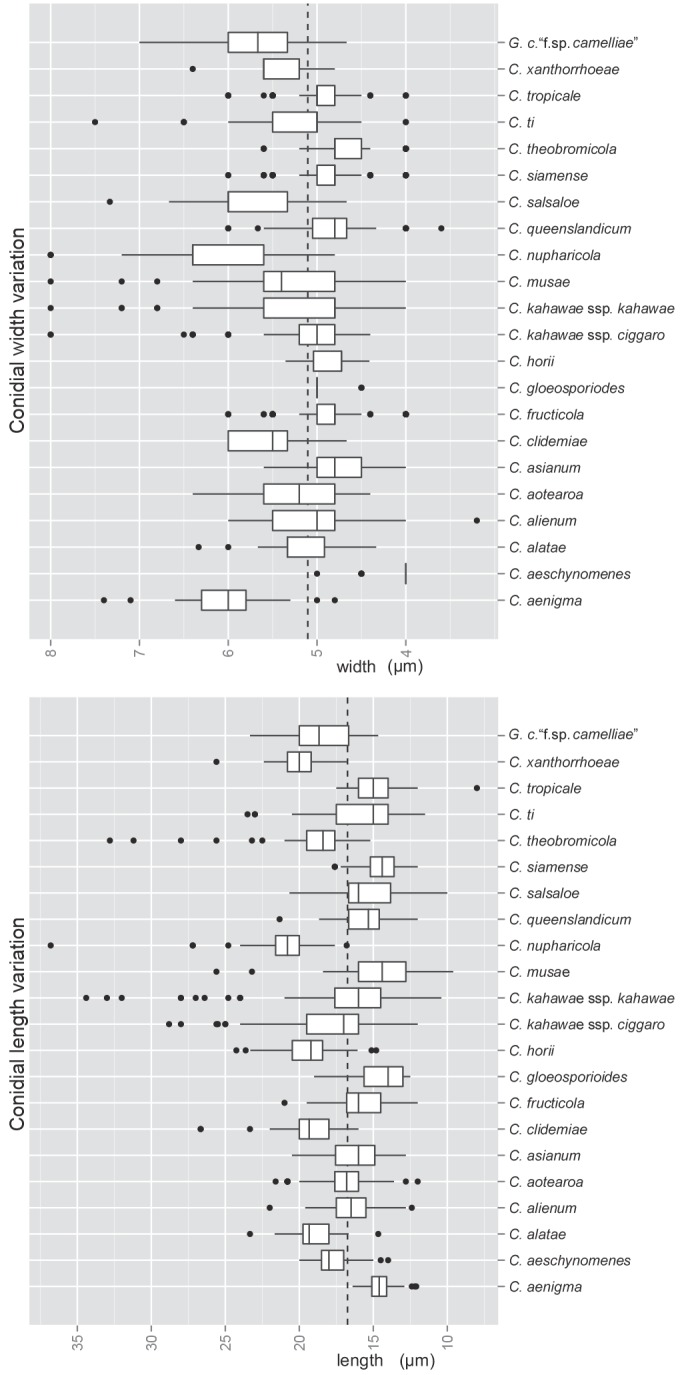
Box plots showing the variation in length and width of conidia produced by the cultures examined in this study. The dashed lines show the mean length (16.74 μm) and width (5.1 μm) across the species complex (n = 1958).
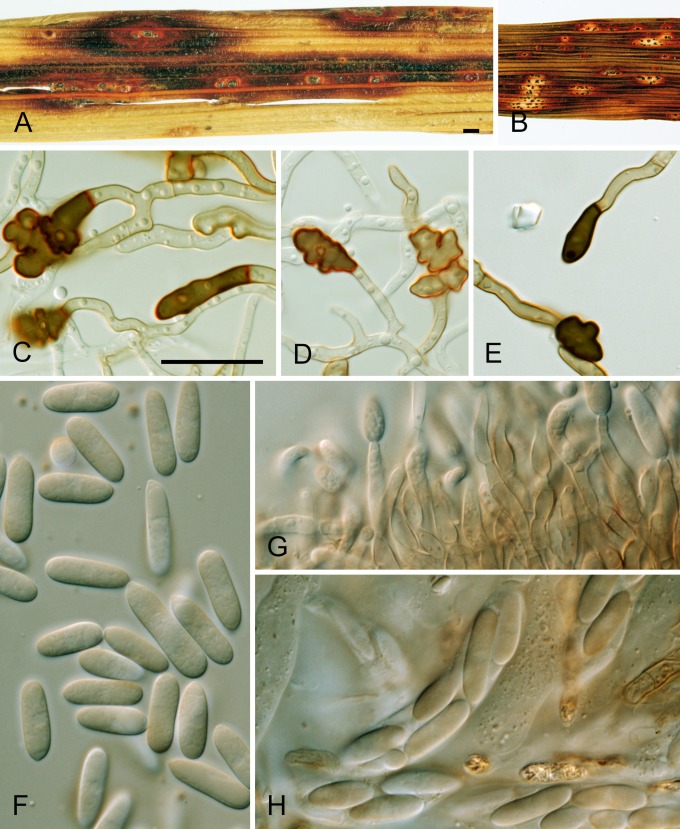
Several species are characterised in part by the development of perithecia in culture. These include four species in the Musae clade (C. alienum, C. fructicola, C. queenslandicum, and C. salsolae) and three in the Kahawae clade (C. clidemiae, C. kahawae subsp. ciggaro, and C. ti). Ascospore size and shape can be a useful species-level diagnostic feature (Fig. 8). In most species the ascospores are strongly curved and typically tapering towards the ends, but in C. clidemiae and C. ti, they are more or less elliptic with broadly rounded ends and not, or only slightly, curved. Individual isolates within any of these species may lose the ability to form perithecia, perhaps associated with cultural changes during storage.
Fig. 8.
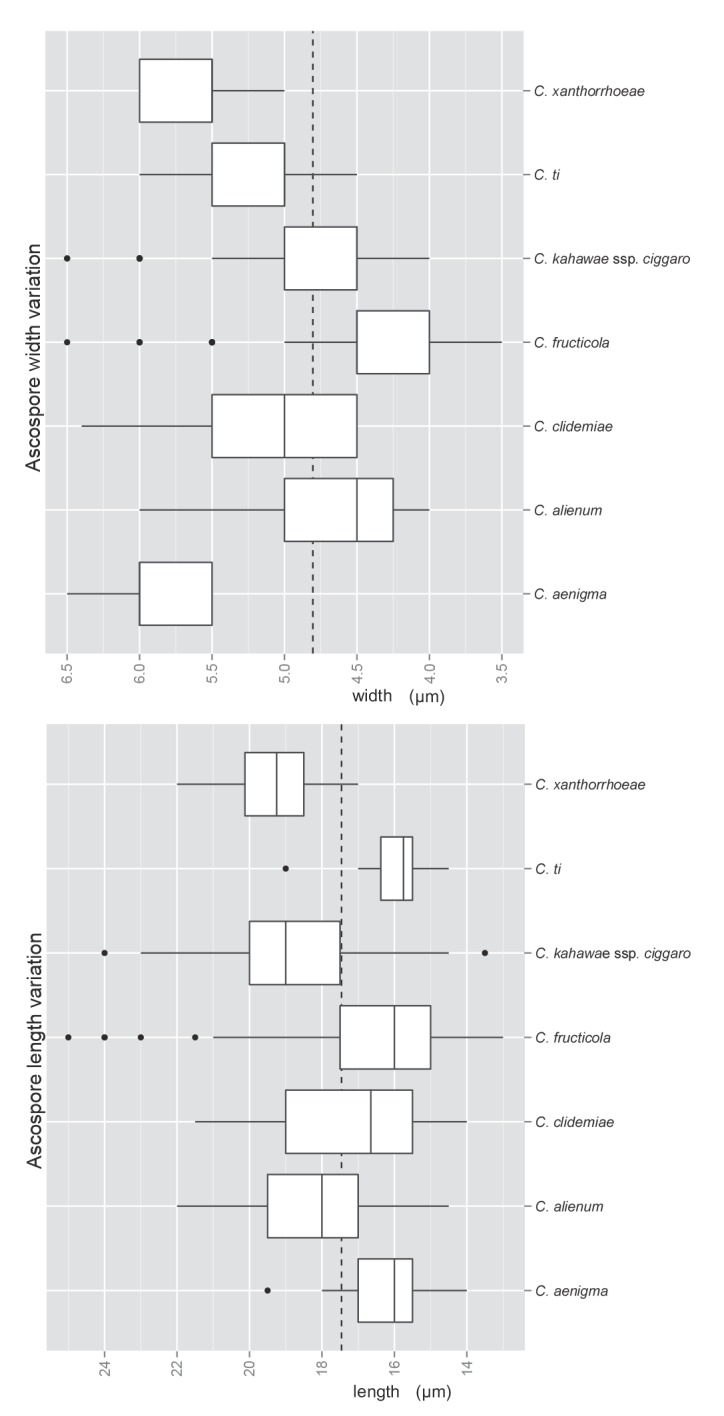
Box plots showing the variation in length and width of ascospores produced by the cultures examined in this study. The dashed lines show the mean length (17.46 μm) and width (4.8 μm) across the species complex (n = 452).
Large, dark-walled stromatic structures are present in the cultures of some species not known to form perithecia. Often embedded in agar, less commonly on the surface or amongst the aerial mycelium, these structures differ from perithecia in comprising a compact tissue of tightly tangled hyphae rather than the pseudoparenchymatous, angular cells typical of perithecial walls. They have a soft, leathery texture compared to the more brittle perithecia. These stromatic structures sometimes develop a conidiogenous layer internally, and following the production of conidia they may split open irregularly, folding back to form a stromatic, acervulus-like structure. These kind of structures are also formed by some species in the C. boninense species complex (Damm et al. 2012b, this issue).
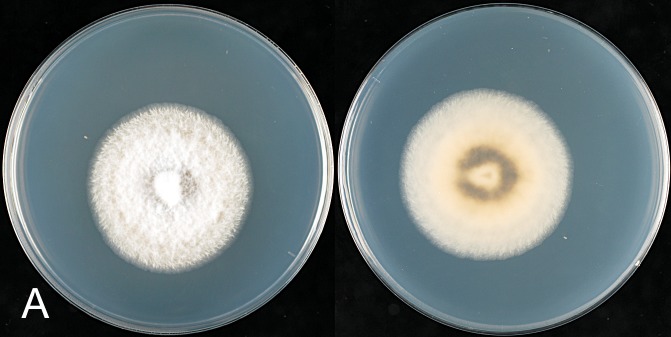
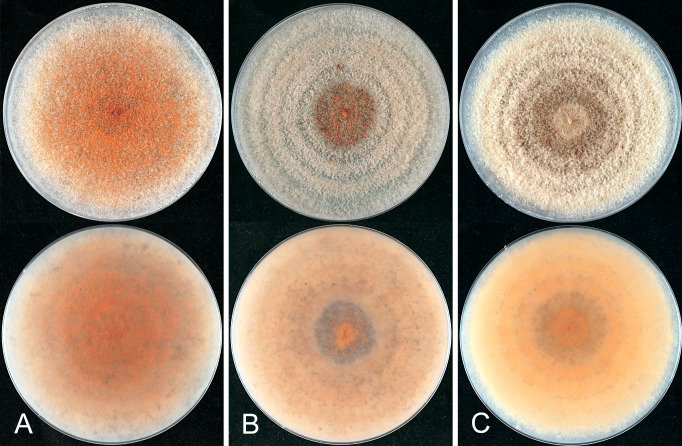
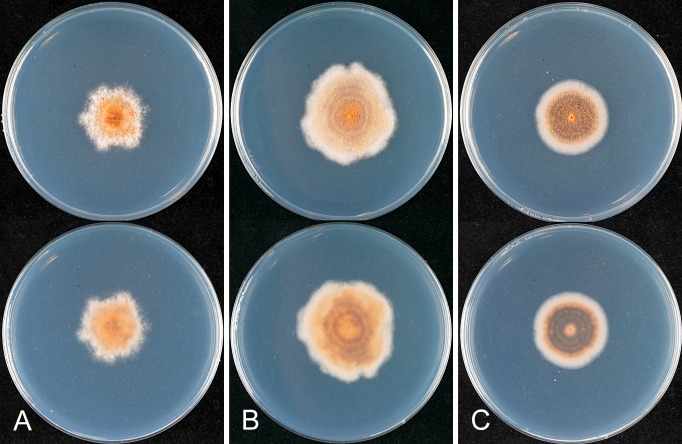
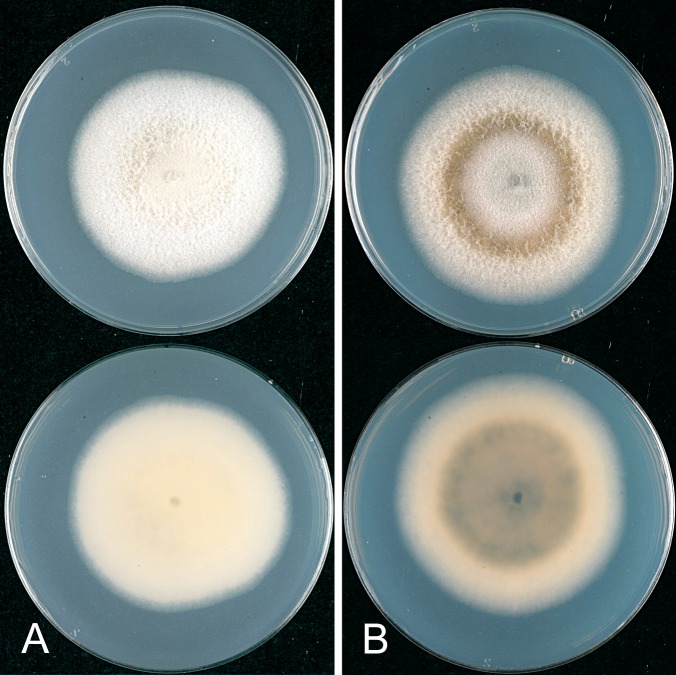
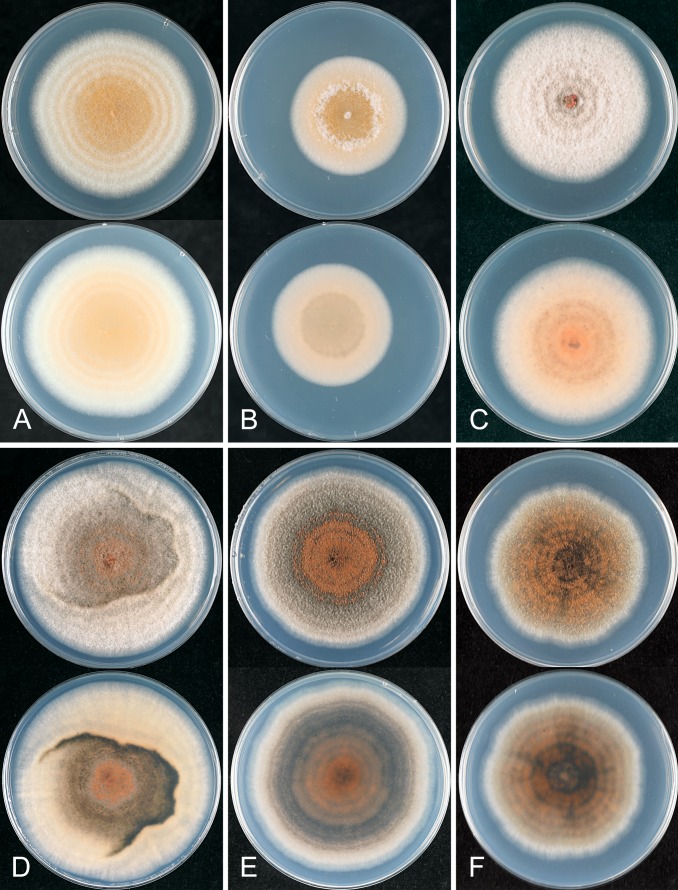
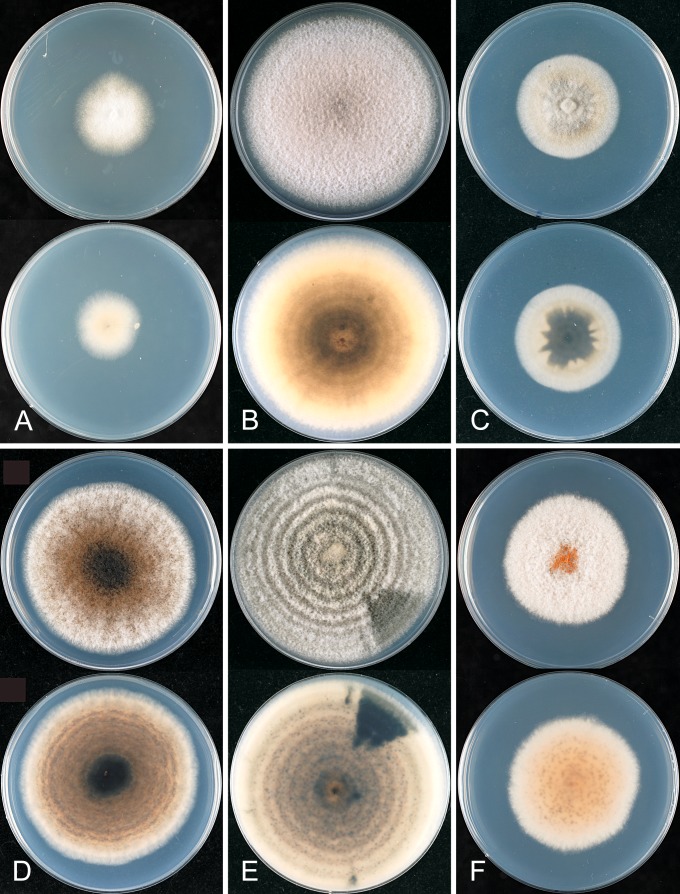
The macroscopic appearance of the cultures is often highly divergent within a species (e.g. C. fructicola and C. theobromicola), in most cases probably reflecting different storage histories of the isolates examined. Prolonged storage, especially with repeated subculturing, results in staling of the cultures, the aerial mycelium often becoming dense and uniform in appearance and colour, and a loss of conidial and perithecial production, and variable in growth rate (Fig. 9). In some species, individual single ascospore or single conidial isolates show two markedly different cultural types, see notes under C. kahawae subsp. ciggaro.
Fig. 9.
A box plot of the diameter of cultures grown on PDA agar at 18 °C for 10 d. The dashed line shows the mean culture size (61.56 mm) across the species complex (n = 719). Note that the data is skewed by some fast growing cultures that reached the agar plate diam (85 mm) in under 10 d.
Some species appear to be host specialised, e.g. C. horii, C. kahawae subsp. kahawae, C. nupharicola, C. salsolae, C. ti, and C. xanthorrhoeae, but those most commonly isolated have broad host and geographic ranges, e.g. C. fructicola, C. kahawae subsp. ciggaro, C. siamense, and C. theobromicola. Colletotrichum gloeosporioides s. str. is commonly isolated from Citrus in many parts of the world, but has been isolated from other hosts as well, such as Ficus, Mangifera, Pueraria, and Vitis. Not all of the species with a broad host range are found everywhere, for example in New Zealand C. alienum is commonly associated with cultivated fruits, whereas species such as C. siamense and C. fructicola, common on these same cultivated fruits in other parts of the world, have not been reported from New Zealand.
Taxonomy
Based on results of the multigene concatenated BI phylogenies, we accept 22 species plus one subspecies within the C. gloeosporioides complex. Isolates authentic for G. cingulata “f. sp. camelliae” form a genetically distinct group, but this is not formally named because of doubts over its relationship to C. camelliae. Based on DNA sequence comparisons, a few other isolates almost certainly represent additional unnamed species. We do not formally describe them because most are known from a single isolate, often stale, with little understanding of either their morphological or biological diversity. In the Musae clade these include ICMP 18614 and ICMP 18616, both from grape from Japan, and ICMP 18726 from pawpaw from the Cook Islands, and in the Kahawae clade ICMP 18699 from chestnut from Japan. These isolates are not included in the phylogenies in this study, but DNA sequences from these isolates have been accessioned into GenBank (ITS: JX009423–JX009428, GAPDH: JX009416–JX009422, ACT: JX009404–JX009407, CAL: JX009408–JX009411, CHS-1: JX009412–JX009415).
Many of the species that we recognise fall into one of two clades, the informally named Musae clade and Kahawae clade (Fig. 2). Each clade contains several species that are phylogenetically well supported in multi-gene analyses, but within the clades branch lengths are short because of the small number of phylogenetically informative characters. This is reflected in the low support values in the gene tree analyses for the species we accept within that clade (Figs 3, 4). Both the Musae and Kahawae clades contain ex-type or authentic cultures from several long accepted species. In this work we have made a pragmatic decision to minimise taxonomic disruption, so that monophyletic subclades within the Kahawae and Musae clades are accepted as species where they include ex-type or authentic cultures. The Musae clade thus includes C. fructicola, C. musae, C. nupharicola, C. siamense, and C. tropicale; and the Kahawae clade includes C. cordylinicola, C. psidii, and C. kahawae. Also belonging in the latter is Glomerella cingulata “f. sp. camelliae”. To provide a consistent taxonomic treatment of the subclades resolved within the Musae and Kahawae clades, several new species and one new subspecies are proposed. In the Musae clade these are C. aenigma, C. aeschynomenes, C. alienum, C. queenslandicum, and C. salsolae; in the Kahawae clade C. aotearoa, C. clidemiae, C. kahawae subsp. ciggaro, and C. ti. The other accepted species, well resolved in all of the gene trees, are C. alatae, C. asianum, C. gloeosporioides, C. horii, C. theobromicola, and C. xanthorrhoeae.
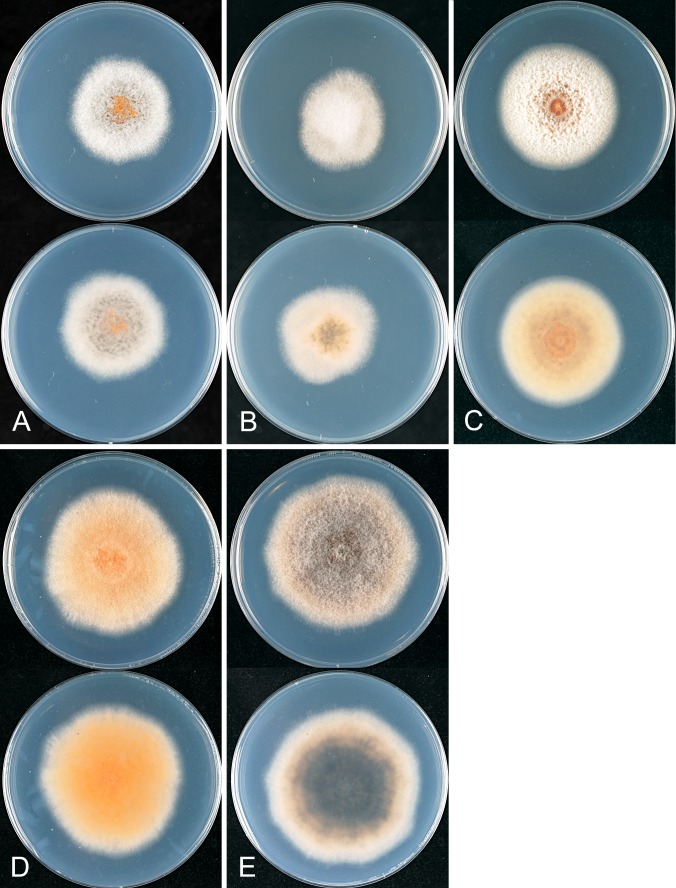
Fig. 4.
A Bayesian inference phylogenetic tree of 32 selected isolates in the Musae clade of the Colletotrichum gloeosporioides species complex. The tree was build using concatenated sequences of the ACT, TUB2, CAL, CHS-1, GAPDH, GS, ITS, and SOD2 genes each with a separate model of DNA evolution. Other details as per Fig.1. B. A species-tree constructed from the same data, the scale is an clock set relative to the last common ancestor of the Musae clade and C. gloeosporioides s. str., as calibrated in Fig. 3.
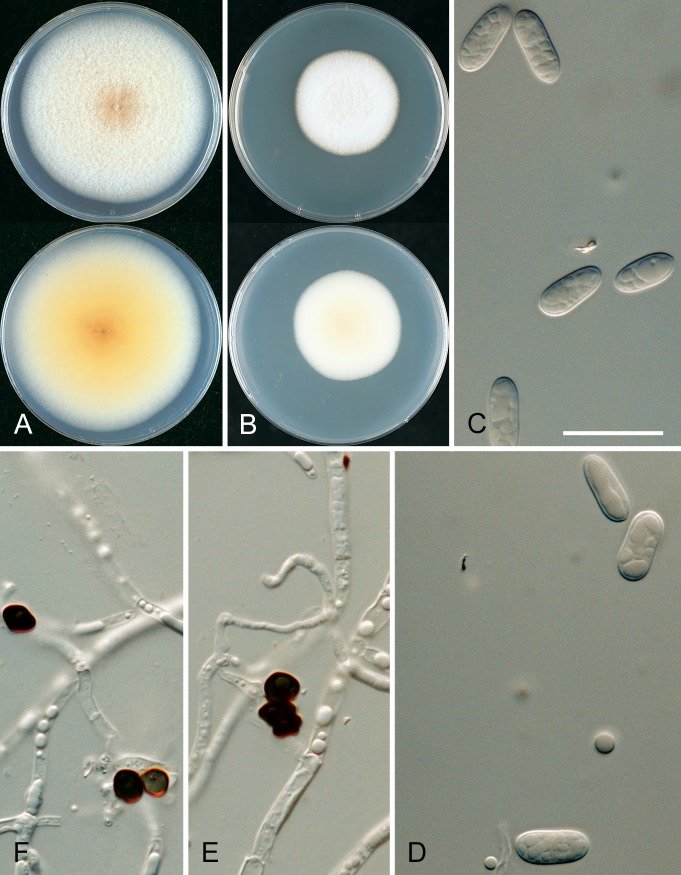
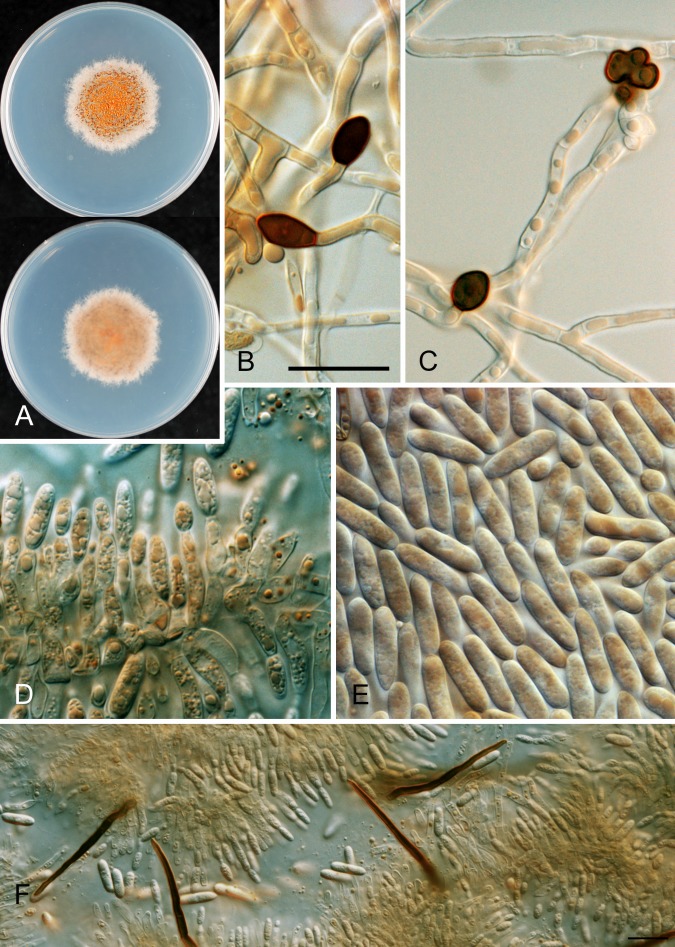
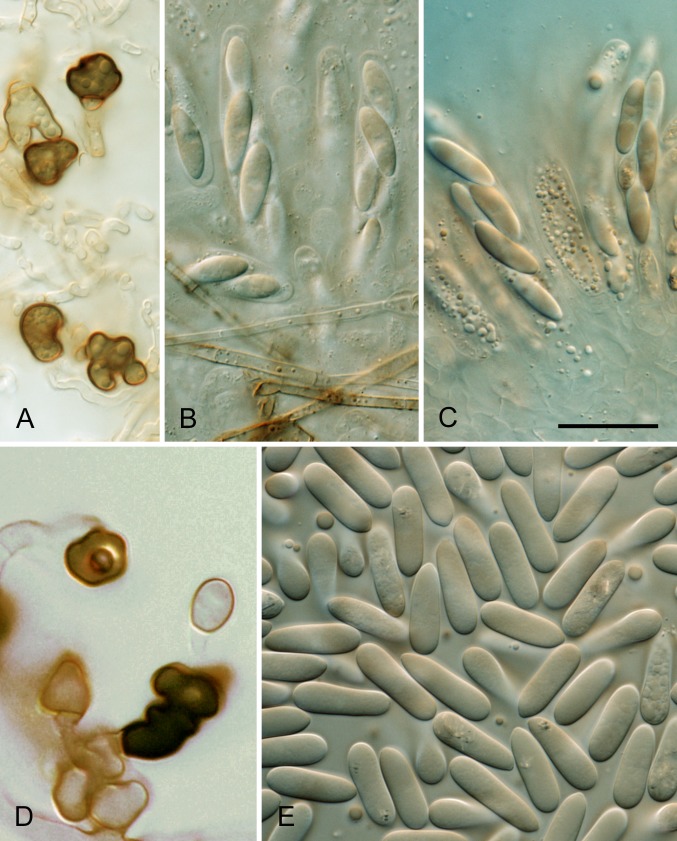
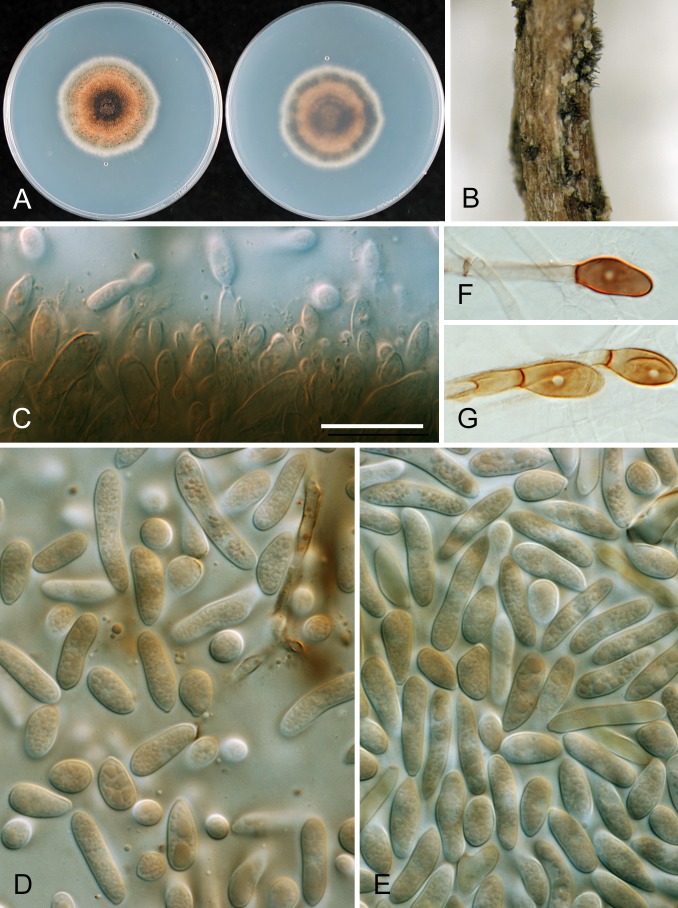
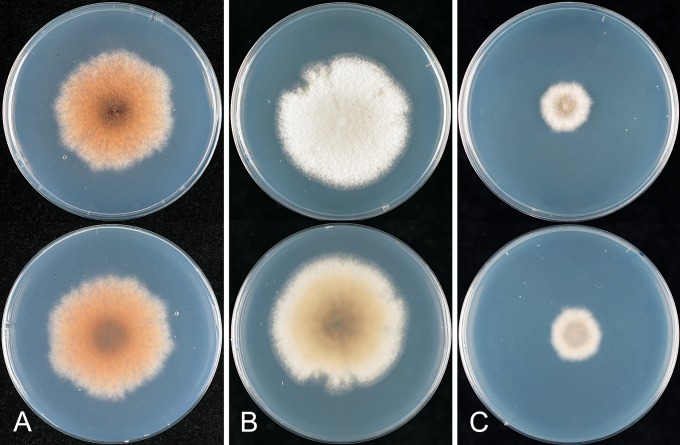
* Colletotrichum aenigma B. Weir & P.R. Johnst., sp. nov. MycoBank MB563759. Fig. 10.
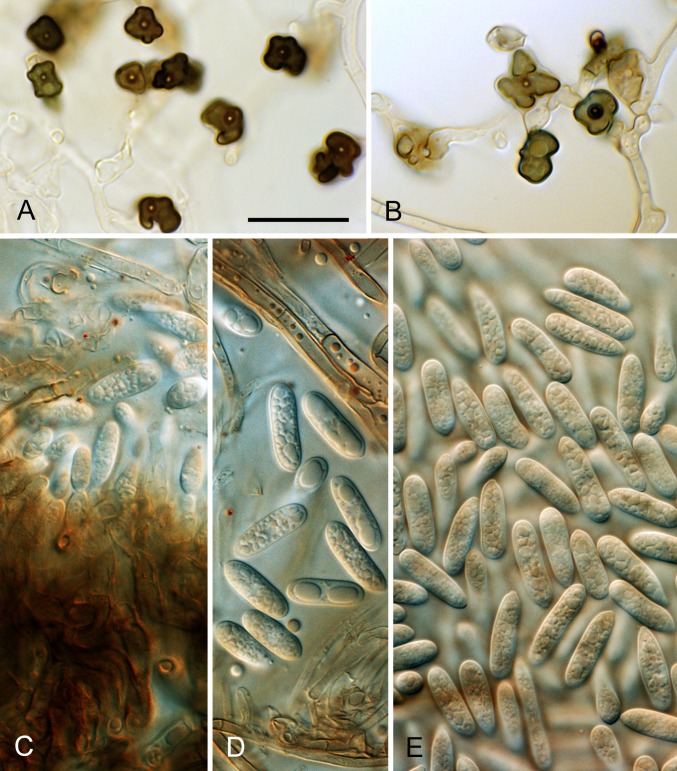
Fig. 10.
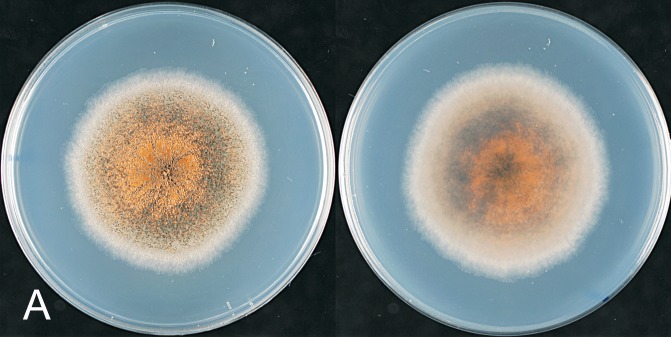
Colletotrichum aenigma. A, C, D, E, F. ICMP 18608 – ex-holotype culture. B. ICMP 18616. A–B. Cultures on PDA, 10 d growth from single conidia, from above and below. C–D. Conidia. E–F. Appressoria. Scale bar C = 20 μm. Scale bar of C applies to C–F.
Etymology: from the Latin aenigma, based on the enigmatic biological and geographic distribution of this species.
Holotype: Israel, on Persea americana, coll. S. Freeman Avo-37-4B, PDD 102233; ex-holotype culture ICMP 18608.
Colonies grown from single conidia on Difco PDA 30–35 mm diam after 10 d. Aerial mycelium sparse, cottony, white, surface of agar uniformly pale orange (7A5) towards centre, more or less colourless towards edge, conidia not associated with well differentiated acervuli and no masses of conidial ooze. In reverse pale orange towards centre. Conidiogenous cells arising haphazardly from dense, tangled hyphae across agar surface, short-cylindric with a poorly differentiated conidiogenous locus. Conidia often germinating soon after release, sometimes forming appressoria, so forming a thin, compact, layer of germinated, septate conidia, germ tubes, and appressoria across the central part of the colony surface. Conidia (12–)14–15(–16.5) × (5–)6–6.5(–7.5) μm (av. 14.5 × 6.1 μm, n = 53), cylindric with broadly rounded ends. Appressoria 6–10 μm diam, subglobose or with a few broad lobes.
Geographic distribution and host range: known from only two collections, one from Pyrus pyrifolia from Japan, the other from Persea americana from Israel.
Genetic identification: ITS sequences are insufficient to separate C. aenigma from C. alienum and some C. siamense isolates. These taxa are best distinguished using TUB2 or GS.
Notes: Although the biology of this species is more or less unknown, it has been found in two widely separate regions and is, therefore, likely to be found to be geographically widespread in the future. Genetically distinct within the Musae clade, this species has a distinctive appearance in culture with sparse, pale aerial mycelium and lacking differentiated acervuli.
Other specimen examined: Japan, on Pyrus pyrifolia, coll. H. Ishii Nashi-10 (ICMP 18686).
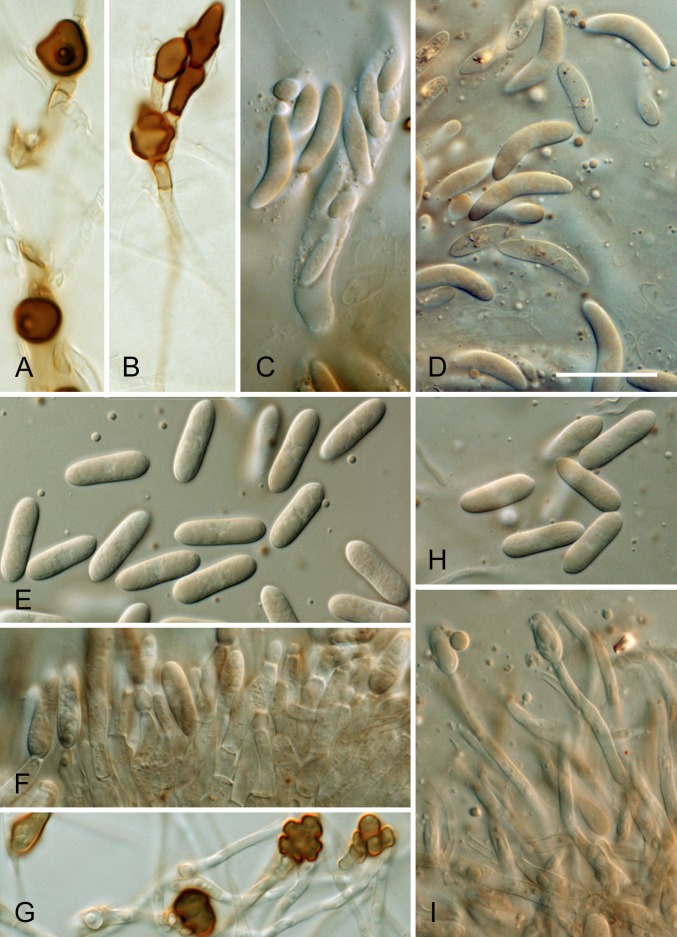
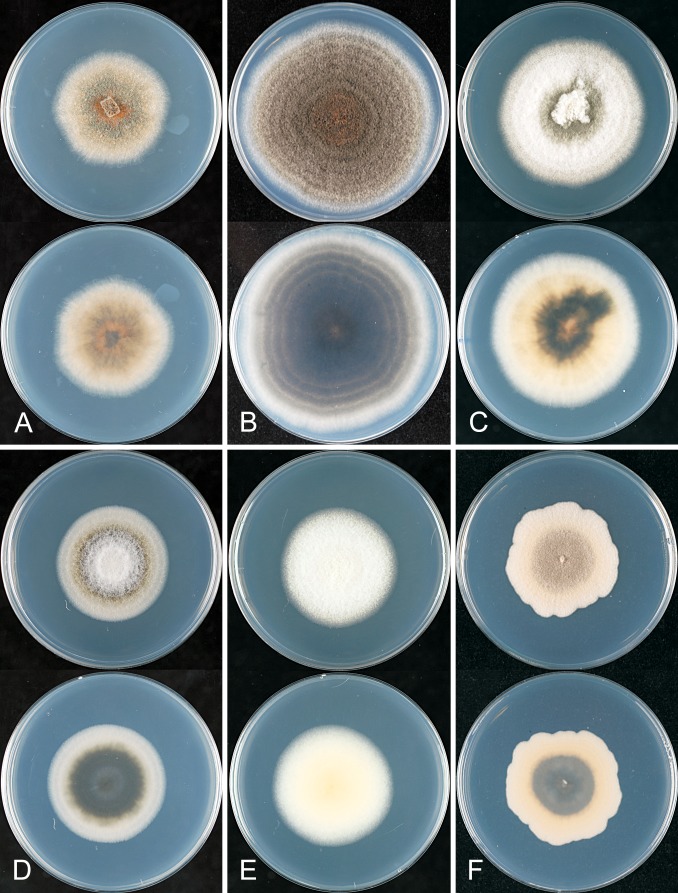
* Colletotrichum aeschynomenes B. Weir & P.R. Johnst., sp. nov. MycoBank MB563590. Fig. 11.
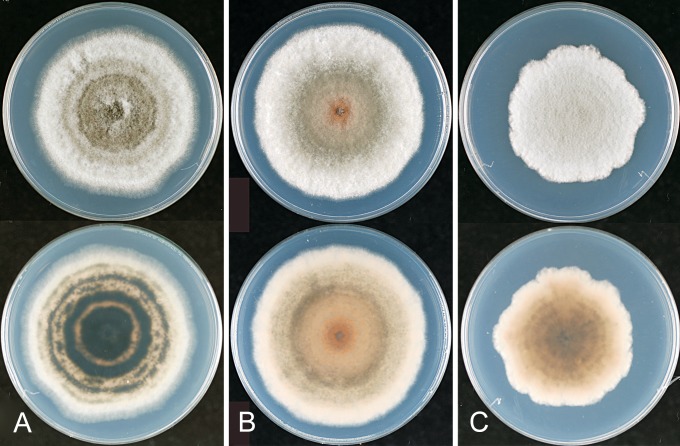
Fig. 11.
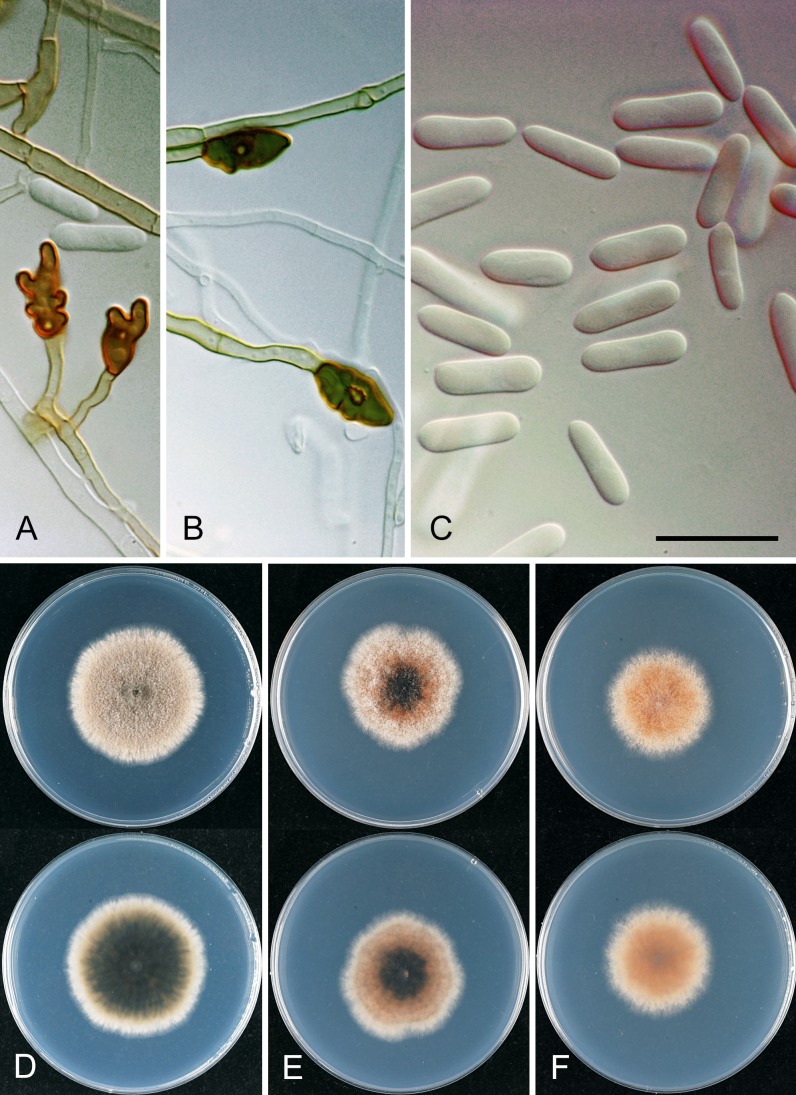
Colletotrichum aeschynomenes. ICMP 17673 – ex-holotype culture. A–C. Appressoria. D. Conidiogenous cells. E. Conidia. F. Cultures on PDA, 10 d growth from single conidia, from above and below. Scale bar of A = 20 μm. Scale bar of A applies to A–E.
= C. gloeosporioides “f. sp. aeschynomenes” (Daniel et al. 1973, as aeschynomene).
Etymology: Based on C. gloeosporioides “f. sp. aeschynomenes”, referring to the host from which this species was originally described.
Holotype: USA, Arkansas, on Aeschynomene virginica stem lesion, coll. D. TeBeest 3-1-3, PDD 101995; ex-type culture ICMP 17673 = ATCC 201874.
Colonies grown from single conidia on Difco PDA 25–35 mm diam after 10 d, aerial mycelium sparse, cottony, white, surface of colony with numerous acervuli, some with dark bases, with orange conidial ooze; in reverse more or less colourless apart from the dark acervuli and orange conidial masses showing through the agar. Conidia (14–)17–18.5(–20) × 4(–5) μm (av. 17.6 × 4.1 μm, n = 30), cylindric, straight, tapering slightly near both ends. Appressoria mostly elliptic to subfusoid, deeply lobed. Perithecia not seen.
Geographic distribution and host range: Reported only from USA, pathogenic to Aeschynomeme.
Genetic identification: ITS sequences do not distinguish C. aeschynomenes from C. fructicola. These taxa are best distinguished using TUB2, GAPDH, or GS.
Notes: Colletotrichum gloeosporioides “f. sp. aeschynomenes” has been used to refer to isolates pathogenic to Aeschynomene virginica, later developed as the weed biocontrol agent Collego (references in Ditmore et al. 2008). It has also been reported from a range of other hosts (TeBeest 1988). Our analyses, based on a single, authentic strain of C. gloeosporioides “f. sp. aeschynomenes” (TeBeest 3.1.3, apparently the source of the single spore isolate originally used in the development of Collego, Ditmore et al. (2008)) show it to be genetically distinct within the Musae clade of the C. gloeosporioides complex. Genetically close to the geographically and biologically diverse C. siamense, it differs morphologically from this species in having slightly longer and narrower conidia which taper slightly toward the ends, and in having larger, strongly lobed appressoria.
An isolate deposited as C. gloeosporioides f. sp. aeschynomenes in CBS (CBS 796.72) by G.E. Templeton, one of the early C. gloeosporioides f. sp. aeschynomenes researchers (Daniel et al. 1973), is genetically distinct to TeBeest 3.1.3 and has been identified by Damm et al. (2012a, this issue) as C. godetiae, a member of the C. acutatum complex. The strain that we examined (Te Beest 3.1.3) matches genetically another strain often cited in the C. gloeosporioides f. sp. aeschynomenes literature (Clar-5a = ATCC 96723) (GenBank JX131331). It is possible that two distinct species, both highly pathogenic to Aeschynomene in Arkansas, have been confused. A survey of additional isolates of Colletotrichum highly virulent to Aeschynomene in Arkansas would clarify the interpretation of the past literature on this pathogen. For example, C. gloeosporioides “f. sp. aeschynomenes” was initially reported as specific to Aeschynomene virginica (Daniel et al. 1973), while later studies reported isolates putatively of the same taxon, to have a wider host range (TeBeest 1988).
Cisar et al. (1994) reported fertile ascospores from crosses between isolates identified as C. gloeosporioides “f. sp. aeschynomenes” and isolates of C. gloeosporioides “f. sp. jussiaeae”, a pathogen of Jussiaea decurrens. The position of C. gloeosporioides “f. sp. jussiaeae” within our phylogeny is not known, but these taxa could prove useful for better understanding of the biological differences between phylogenetically defined species of Colletotrichum.
Specimen examined: USA, Arkansas, on Aeschynomene virginica stem lesion, coll. D. TeBeest 3.1.3 (ICMP 17673 = ATCC 201874).
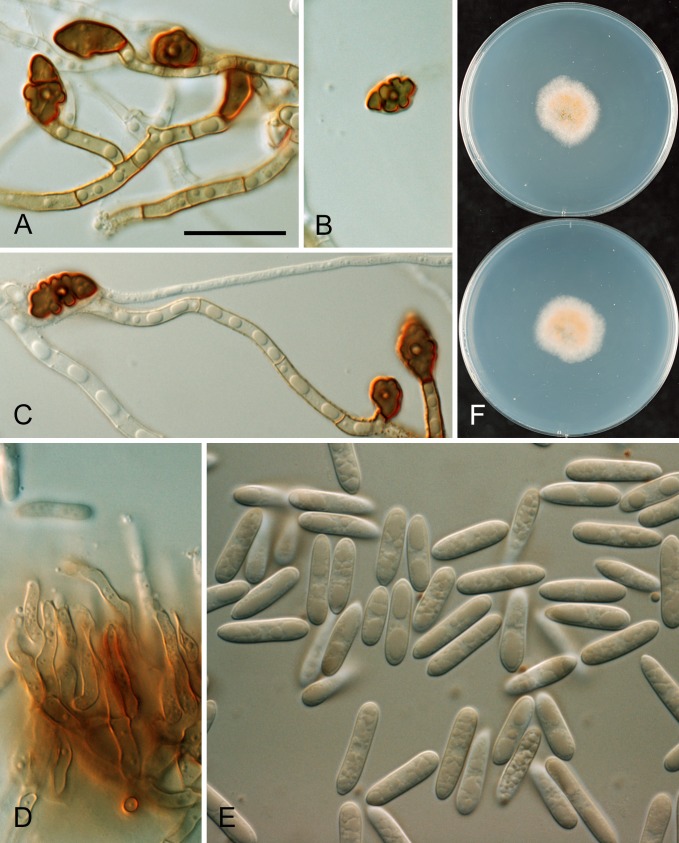
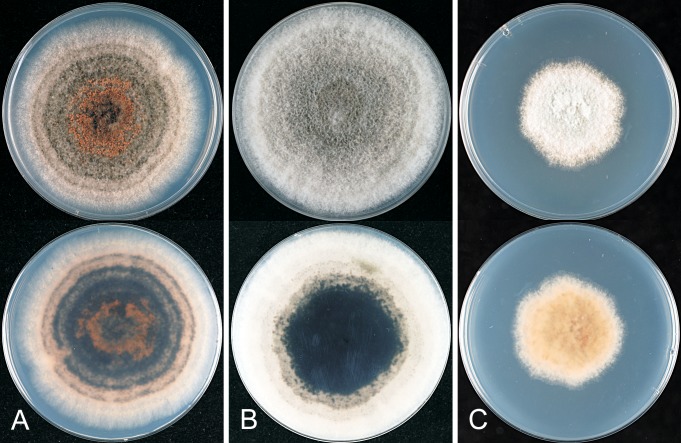
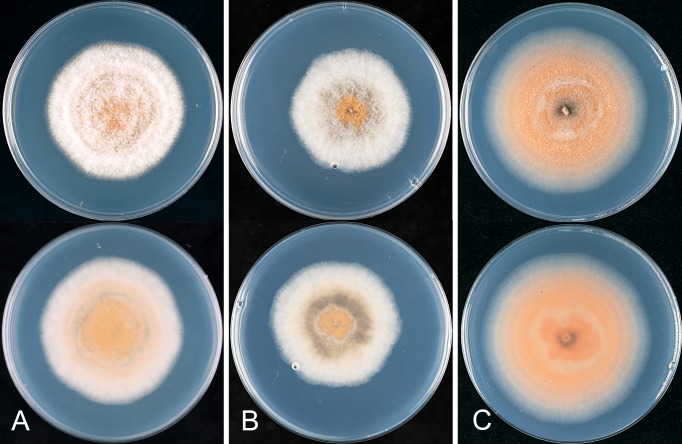
* Colletotrichum alatae B. Weir & P.R. Johnst., sp. nov. MycoBank MB563747. Fig. 12.
Fig. 12.
Colletotrichum alatae. ICMP 18122. A. Cultures on PDA, 10 d growth from single conidia, from above and below. B–C. Appressoria. D. Conidiogenous cells and conidia. E. Conidia. F. Setae. Scale bars B, F = 20 μm. Scale bar of B applies to B–E.
= Colletotrichum gloeosporioides “f. alatae” R.D. Singh, Prasad & R.L. Mathur, Indian Phytopathol. 19: 69. 1966. [nom. inval., no Latin description, no type designated].
Etymology: Based on the invalid name C. gloeosporioides “f. alatae” (Singh et al. 1966), referring to Dioscorea alata, the scientific name for yam.
Holotype: India, Rajasthan, Udaipur, on Dioscorea alata leaves and stems, coll. K.L. Kothari & J. Abramham, 1959, CBS H-6939; ex-type culture and putatively authentic isolate of C. gloeosporioides f. alatae CBS 304.67 = ICMP 17919.
Colonies grown from single conidia on Difco PDA 30–40 mm diam after 10 d. Ex-holotype culture looks “stale”, with low, felted, dense, pale grey aerial mycelium, orange agar surface showing through near the margin, scattered dark based acervuli with orange conidial masses near centre; in reverse deep pinkish orange with patches of grey pigment near centre. ICMP 18122 with aerial mycelium sparse, colony surface with numerous discrete, dark-based acervuli with bright orange conidial ooze, margin of colony feathery; in reverse irregular sectors with pale grey pigment within the grey, otherwise colourless apart from the colour of the acervuli and conidial masses. Conidia (14.5–)18–19.5(–23.5) × (4.5–)5–5.5(–6.5) μm (av. 18.9 × 5.2 μm, n = 40), cylindric, straight, ends rounded, a few tapering towards the basal end. Appressoria mostly simple, elliptic to fusoid in shape, sometime developing broad, irregular lobes, about 7–13.5 × 5–10.5 μm. Perithecia not seen.
Geographic distribution and host range: Known only from yam (Dioscorea alata), from Nigeria, Barbardos, India, Guadeloupe.
Genetic identification: ITS sequences distinguish C. alatae from all other taxa.
Notes: Anthracnose diseases of yam are found throughout the regions where the host is grown (e.g. Winch et al. 1984, Prasad & Singh 1960, Singh et al. 1966, Abang et al. 2002, 2003). Isolates from diseased yam leaves are morphologically (Winch et al. 1984) and genetically (Abang et al. 2002) diverse. Both of these authors used a broad species concept, grouping all isolates sourced from yam under the single name C. gloeosporioides. In this paper we accept part of that diversity to represent a distinct species, newly described here as C. alatae. The type specimen of C. alatae matches the SGG (slow growing grey) group of Abang et al. (2002), the group that these authors found to be more pathogenic to yam than the other morphological and genetic groups they recognised within C. gloeosporioides. In addition to the Nigerian isolates of Abang et al. (2002), isolates from yam from Barbados (isolates SAS8 and SAS9 from Sreenivasaprasad et al. 1996), Guadeloupe (GenBank accession GQ495617) and India (CBS 304.67 and GenBank accession FJ940734) belong in this clade, while no isolates from other hosts have been found.
Other isolates from yam that we sequenced included some representing the Abang et al. (2002) FGS group (Abang Cg22 = ICMP 18120, Abang Cg13 = ICMP 18125, Abang CgS6 = ICMP 18117, Abang CgS2 = ICMP 18121), a group distinguished from the highly pathogenic SGG isolates by faster growth in culture and shorter conidia (Abang et al. 2002). Two of these isolates (ICMP 18120, 18125) genetically match C. fructicola, the others match C. siamense.
Several names have been applied to Colletotrichum specimens from anthracnose of yam stems and leaves, including Gloeosporium pestis Massee, G. “dioscoreae” Sawada (nom. inval.; no Latin diagnosis), Colletotrichum dioscoreae Av.-Saccá 1917, and C. dioscoreae Tehon 1933. In addition, Gloeosporium bomplandii Speg. was described from a host doubtfully identified as Dioscorea. Because of the broad genetic diversity of Colletotrichum spp. associated with diseased yam, the lack of cultures from any of these early type specimens, and the uncertainty to which part of the yam-associated diversity they correspond, we have chosen not to use these names for our newly recognised, yam-specialised pathogen. Whether the post-harvest tuber rot referred to as dead skin disease of yam (Abang et al. 2003, Green & Simmons 1994) is caused by the same Colletotrichum population as associated with diseased foliage is not known.
Other specimen examined: Nigeria, Kpite, on Dioscorea alata leaf, coll. M.M. Abang Cg25, 2001 (ICMP 18122).
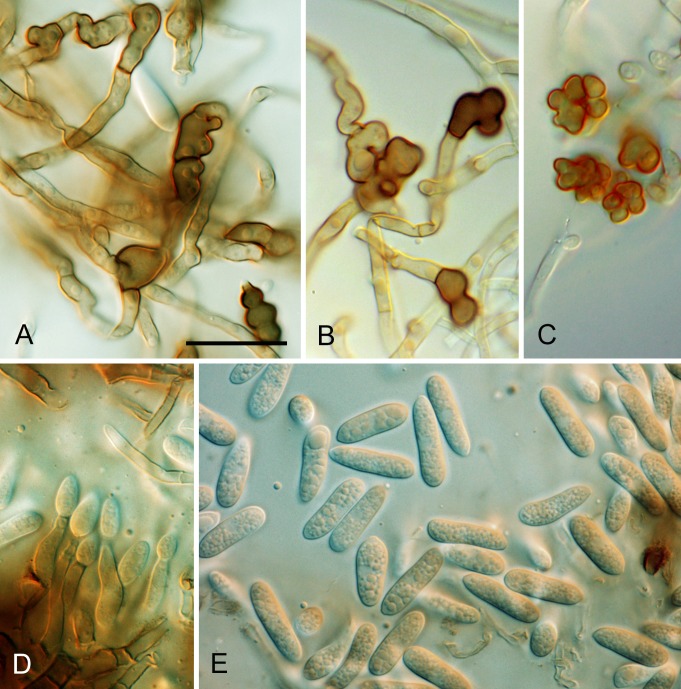
* Colletotrichum alienum B. Weir & P.R. Johnst., sp. nov. MycoBank MB563591. Figs 13, 14.
Fig. 13.
Colletotrichum alienum. A, E, F. ICMP 12071 – ex-holotype culture. B. ICMP 18703. C–D. ICMP 12068. G–I. ICMP 18691 (ex DAR 37820). A–B. Appressoria. C–D. Asci and ascospores. E. Conidia. F. Conidiogenous cells. G. Appressoria. H. Conidia. I. Conidiogenous cells. Scale bar D = 20 μm. Scale bar of D applies to A–I.
Fig. 14.
Colletotrichum alienum. A. ICMP 12071 – ex-holotype culture. B. ICMP 12068. C. ICMP 18691 (ex DAR 37820). A–C. Cultures on PDA, 10 days growth from single conidia, from above and below.
Etymology: Based on the biology of this species, confined to exotic hosts and presumed to be a recent introduction to Australasia.
Holotype: New Zealand, Auckland, Kumeu research orchard, Malus domestica fruit rot, coll. P.R. Johnston C824, 14 Aug. 1987, PDD 101996; ex-type culture ICMP 12071.
Colonies grown from single conidia on Difco PDA 85 mm diam after 10 d. Colonies often with distinct sectors; some with cottony, grey aerial mycelium with numerous dark-based acervuli and orange conidial ooze visible through the mycelium; others with dense, cottony to felted mycelium, fewer acervuli and these hidden by the dense mycelium. In reverse, irregular dark grey patches and sectors masking the pale orange coloured pigmentation. ICMP 18691 looks “stale” with slow growth, dense, pale aerial mycelium and sparse conidial production and no perithecia. Conidia (12.5–) 15.5–17.5(–22) × (3–)5–5.5(–6) μm (av. 16.5 × 5.0 μm, n = 70), cylindric with broadly rounded ends. Appressoria mostly simple, globose to short-cylindric, a few with broad, irregular lobes; ICMP 18691 has mostly lobed appressoria. Perithecia forming in most cultures after about 10 d, dark-walled, globose with short, narrow ostiolar neck. Ascospores (14.5–)17–19.5(–22) × 4–5(–6) μm (av. 18.1 × 4.6 μm, n = 55), cylindric, curved, tapering slightly to each end.
Geographic distribution and host range: Known only from Australia and New Zealand, common on a wide range of introduced fruit crops.
Genetic identification: ITS sequences do not separate C. alienum from some C. siamense isolates. These taxa are best distinguished using CAL or GS.
Notes: Common on commercial fruit crops, this fungus was referred to as C. gloeosporioides Group A by Johnston & Jones (1997) and Johnston et al. (2005).
Other specimens examined: Australia, New South Wales, Murwillumbah, on Persea americana (DAR 37820 = IMI 313842 = ICMP 18691). New Zealand, Auckland, Oratia, Shaw Rd, on Malus domestica fruit rot, coll. P.R. Johnston C938.5, 14 Apr. 1988 (ICMP 18725); Bay of Plenty, Katikati, on Diospyros kaki ripe fruit rot, coll. M.A. Manning, Jun. 1989 (ICMP 17972); Bay of Plenty, Te Puke, on Persea americana ripe fruit rot, coll. W.F.T. Hartill, 2 Feb. 1988 (ICMP 18704); Bay of Plenty, Te Puna, on Persea americana ripe fruit rot, coll. W.F.T. Hartill, 25 Jan. 1988 (ICMP 18703); Bay of Plenty, on Persea americana ripe fruit rot, coll. W.F.T. Hartill, Feb. 1991 (ICMP 18621); Waikato, Hamilton, on Malus domestica fruit rot, coll. G.I. Robertson, May 1988 (ICMP 12068).
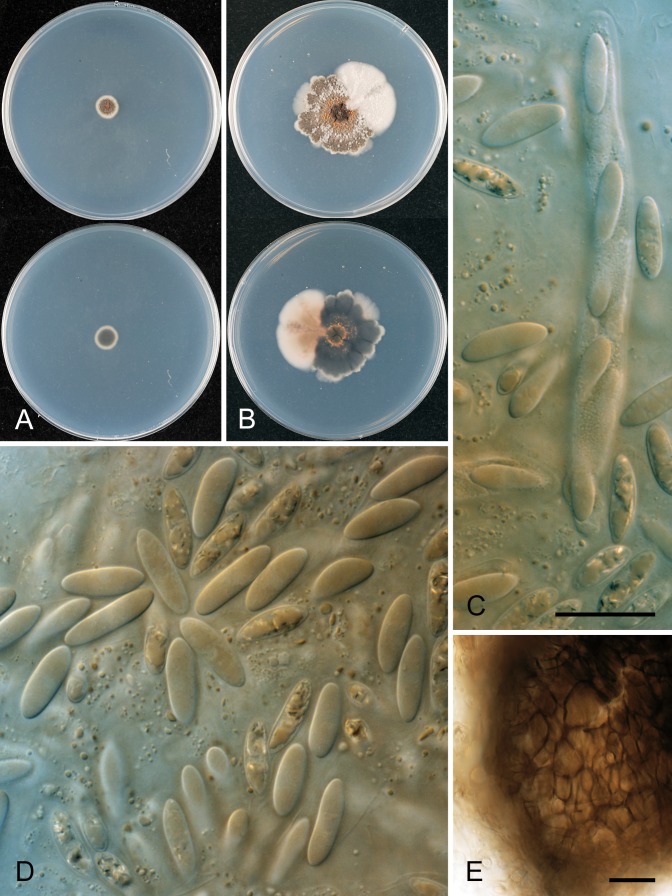
* Colletotrichum aotearoa B. Weir & P.R. Johnst., sp. nov. MycoBank MB800213. Figs 15, 16.
Fig. 15.
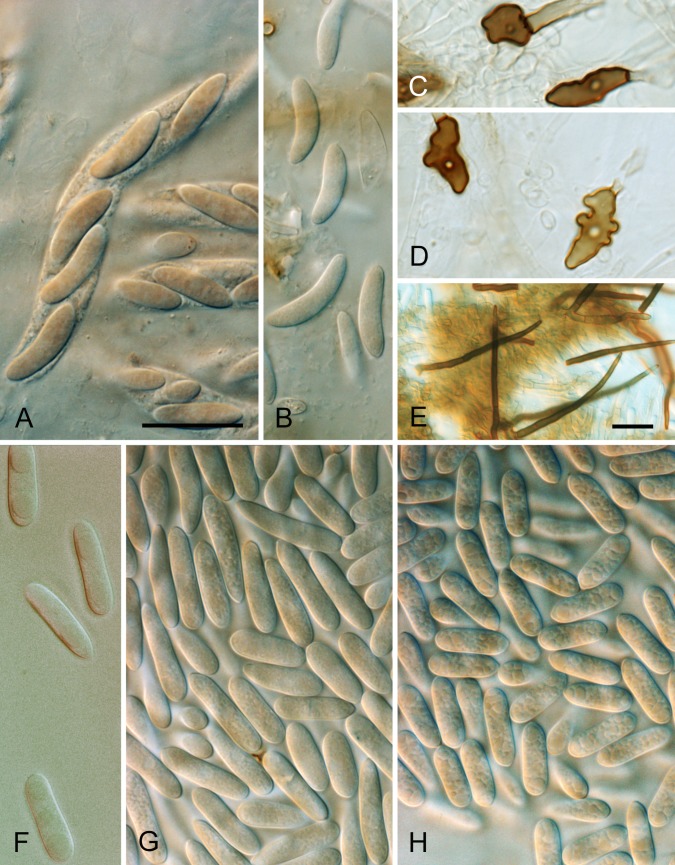
Colletotrichum aotearoa. A. ICMP 17324. B. ICMP 18529. C. ICMP 18548. D. 18532. E. ICMP 18540. A–C. Appressoria. D. Conidiogenous cells. E. Conidia. Scale bar A = 20 μm. Scale bar of A applies to A–E.
Fig. 16.
Colletotrichum aotearoa. A. ICMP 18537 – ex-holotype culture. B. ICMP 18548. C. ICMP 18532. D. ICMP 18740. E. ICMP 18533. F. ICMP 18530. A–F. Cultures on PDA, 10 d growth from single conidia, from above and below.
Etymology: Based on the Maori name for New Zealand; most isolates from native New Zealand plants belong here.
Holotype: New Zealand, Auckland, Glen Innes, Auckland University campus, on Coprosma sp. incubated berries, coll. B. Weir C1282.4, 30 Apr 2009, PDD 101076; ex-type culture ICMP 18537.
Colonies grown from single conidia on Difco PDA 70–85 mm diam after 10 d, several isolates with restricted growth, 50–55 mm diam with an irregularly scalloped margin. Aerial mycelium cottony to dense cottony, tufted near centre, grey to dark grey, scattered, small, dark-based acervuli and large, globose, stromatic structures partially embedded in agar, these sometimes splitting apart and forming conidia. In reverse typically with pinkish-orange pigments, variable in intensity, in some isolates this colour partially hidden by more or less concentric bands of dark grey pigment. Conidia (12–)16–17.5(–21.5) × (4.5–)5–5.5(–6.5) μm (av. 16.9 × 5.2 μm, n = 216), cylindric, straight, apex broadly rounded, often tapering slightly towards subtruncate base, 0-septate, hyaline. Appressoria variable in shape, simple to broadly lobed, sometimes in groups, sometimes intercalary, about 7–17 × 4–9.5 μm. Perithecia not seen in culture.
Geographic distribution and host range: Confirmed only from New Zealand, but GenBank records suggest C. aotearoa also occurs in China (see below). In New Zealand this species is common on a taxonomically diverse set of native plants, as both a fruit rot and a leaf endophyte, and has also been isolated from leaves of several species of naturalised weeds.
Genetic identification: ITS sequences do not separate C. aotearoa from several taxa in the Kahawae and Musae clades. This species can be distinguished using several other genes, including TUB2, CAL, GS, and GAPDH.
Notes: All isolates in the C. gloeosporioides complex from New Zealand native plants studied here belong in the Kahawae clade, and most of these are C. aotearoa; a small number of leaf endophyte isolates from New Zealand native trees are C. kahawae subsp. ciggaro. The C. aotearoa isolates have been isolated as endophytes from symptomless leaves as well as from rotting fruit from native trees. Morphologically indistinguishable from isolates of C. kahawae subsp. ciggaro, this species is distinguished genetically with all genes sampled, except ITS. The GAPDH gene tree splits C. aotearoa into two well supported clades, but these do not correlate to any other features, either geographic or biological. Isolates associated with distinctive and common leaf spots on Meryta sinclairii, first recorded by Beever (1984), belong in this species. Whether isolates of C. aotearoa from other hosts are able to cause the same disease on Meryta is not known.
Also in C. aotearoa are a range of isolates from weeds that have become naturalised in New Zealand. We assume that C. aotearoa is a New Zealand native species. It has a broad host range amongst native plants and has apparently jumped host to some weeds. It has never been found associated with cultivated plants or as a rot of cultivated fruit.
Colletotrichum aotearoa may also occur in China. ITS sequences from isolates from Boehmeria from China (GenBank records GQ120479 and GQ120480) from Wang et al. (2010) match exactly a set of C. aotearoa isolates. ITS between-species differences within the C. gloeosporioides complex are very small, so this match needs confirming with additional genes. C. aotearoa was referred to as Undescribed Group 2 by Silva et al. (2012b).
Other specimens examined: New Zealand, Auckland, Freemans Bay, on Vitex lucens fruit, coll. P.R. Johnston C1252.1, 26 Aug. 2007 (ICMP 18532; PDD 92930). on Berberis sp. leaf spot, coll. N. Waipara C69 (ICMP 18734); Auckland, Mangere, on Berberis glaucocarpa leaf spot, coll. N. Waipara C7, Jun. 2007 (ICMP 18528); Auckland, Waitakere Ranges, on Kunzea ericoides leaf endophyte, coll. S. Joshee 7Kun3.5, Jan. 2004 (ICMP 17324); Auckland, Waitakere Ranges, on Prumnopitys ferruginea leaf endophyte, coll. S. Joshee 8Mb5.1, Jan. 2004 (ICMP 18533); Auckland, Waitakere Ranges, on Dacrycarpus dacrydioides leaf endophyte, coll. S. Joshee 5K5.9, Jan. 2004 (ICMP 18535); Auckland, St Johns, Auckland University campus, on Coprosma sp. incubated berries, coll. B. Weir C1282.1, 30 Apr. 2009 (ICMP 18577); Auckland, Mt Albert, on Acmena smithii lesions fruit, coll. P.R. Johnston C847, 9 Sep. 1987 (ICMP 18529); Auckland, Glen Innes, Auckland University campus, on Coprosma sp. incubated berries, coll. B. Weir C1282.3, 30 Apr. 2009 (ICMP 18536); Auckland, Orakei, on Ligustrum lucidum leaf spot, coll. C. Winks & D. Than M136.3 (ICMP 18748); Auckland, Waitakere Ranges, on Podocarpus totara leaf endophyte, coll. S. Joshee 3T5.6, Jan. 2004 (ICMP 17326); Auckland, Waitakere Ranges, Huia, on Geniostoma ligustrifolium leaf endophyte, coll. S. Bellgard M128, 8 Jul. 2010 (ICMP 18540); Auckland, Waitakere Ranges, Huia, on Coprosma sp. rotten berry, coll. S. Bellgard M130-2, 8 Jul. 2010 (ICMP 18541); Auckland, Waiheke Island, Palm Beach, on Meryta sinclairii leaf spot, coll. P.R. Johnston C1310.1, 21 Mar. 2010 (PDD 99186; ICMP 18742); Auckland, Tiritiri Island, on Dysoxylum spectabile fruit rot, coll. P.R. Johnston C1220, 12 Feb. 1997 (PDD 67042; ICMP 18740); Northland, Whangaruru, on Vitex lucens fruit rot, coll. P.R. Johnston C880.1, L. Brako, P. Berry, 28 Jan. 1988 (PDD 48408; ICMP 18530); on Berberis sp. leaf spot, coll. N. Waipara C77 (ICMP 18735), on Lonicera japonica leaf spot, coll. N. Waipara J3 (ICMP 18736); Wellington, Waikanae, on Coprosma sp. leaf, coll. B. Weir C1285, 14 May 2009 (ICMP 18548); Auckland, Wenderholm Regional Park, on Melicytus ramiflorus leaf endophyte, coll. G.C. Carroll MELRA, 16 Sep. 2009 (ICMP 18543).
* Colletotrichum asianum Prihastuti, L. Cai & K.D. Hyde, Fungal Diversity 39: 96. 2009. Fig. 17.
Fig. 17.
Colletotrichum asianum. A. ICMP 18648 (ex CBS 124960). B. ICMP 18580 (ex MFLU 090234). C. ICMP 18603 (ex MAFF 306627). D. ICMP 18604 (ex HKUCC 18602). E. ICMP 18696 (ex IMI 313839). A–E. Cultures on PDA, 10 d growth from single conidia, from above and below.
Prihastuti et al. (2009) provide a description of this species.
Geographic distribution and host range: Known on Mangifera indica from Australia, Colombia, Japan, Panama, Philippines, and Thailand; also reported on Coffea arabica from Thailand.
Genetic identification: Colletotrichum asianum is distinguished from all other taxa using any of the genes tested, including ITS.
Notes: Although the type specimen is from coffee, this fungus is isolated commonly from mango (Mangifera indica) (e.g. Morphological Group 1 from Than et al. 2008; IMI 313839 from Australia; MAFF 306627 from Japan). Isolates referred to Colletotrichum indet. sp. 1 by Rojas et al. (2010), also associated with mango fruit rots, again match C. asianum. Based on ITS sequences, isolates Man-63 and Man-69 cited by Afanador-Kafuri et al. (2003) from mango from Colombia, are probably also C. asianum. Several papers have reported genetically uniform populations of C. gloeosporioides associated with M. indica around the world (e.g. Hodson et al. 1993, Alahakoon et al. 1994, Sanders & Korsten 2003) and these perhaps also represent C. asianum, although DNA sequences are not available to confirm this.
Three earlier species, originally described from leaves rather than fruit of Mangifera, may provide earlier names for C. asianum but type material for these species has not been examined in this study; C. mangiferae Kelkar, Gloeosporium mangiferae Henn. 1898, and G. mangiferae Racib. 1900. As with most substrates, several different species of Colletotrichum often occur on the same host. For example, Damm et al. (2012a, b, this issue) report members of the C. acutatum and C. boninense species complexes, C. simmondsii, C. fioriniae, and C. karstii, from mango from Australia.
Isolates from Capsicum reported by Than et al. (2008) as C. gloeosporioides Morphological Group 2 (e.g. isolates Ku4 = ICMP 18575 and Ku8 = ICMP 18618), were referred to as C. asianum by Hyde et al. (2009), however they are genetically distinct from C. asianum and belong to C. siamense based on our analyses.
The C. asianum protologue designates the holotype as MFLU 090234, and the culture derived from the holotype as “BCC” with no strain number. The ex-holotype culture is listed as BDP-I4 in the Prihastuti et al. (2009) Table 1, but this number is not mentioned in the description. Culture BDP-I4 was obtained from the authors (Prihastuti et al. 2009) for this study.
Specimens examined: Australia, New South Wales, Sextonville, on Mangifera indica, 1987 (IMI 313839 = ICMP 18696). Philippines, on Mangifera indica (MAFF 306627 = ICMP 18603). Thailand, Chiang Mai, on Mangifera indica fruit, coll. P.P. Than M3 (HKUCC 10862 = ICMP 18605); Chiang Mai, on Mangifera indica fruit, coll. P.P. Than M4 (HKUCC 10863 = ICMP 18604); Mae Lod Village, Mae Taeng District, Chiang Mai, on Coffea arabica berries, coll. H. Prihastuti BPD-I4, 16 Jan. 2008 (ex-holotype culture of C. asianum from specimen MFLU 090234 = ICMP 18580 = CBS 130418). Panama, Gamboa, on Mangifera indica fruit rot, coll. S. Van Bael GJS 08-144, Jul 2008 (CBS 124960 = ICMP 18648).
Colletotrichum boehmeriae Sawada, Hakubutsu Gakkwai Kwaihô (Trans. Nat. Hist. Soc. Formosa) 17: 2. 1914.
Notes: Sawada (1922) provided an English translation of his original description. This species was described as a stem pathogen of Boehmeria nivea, and remains in use in this sense (e.g. Li & Ma 1993). Wang et al. (2010) cite several GenBank accessions from isolates they identify as C. gloeosporioides that cause severe disease of Boehmeria. Based on a comparison of the GenBank data with our ITS gene tree, these and other isolates from the same host deposited by the same authors (GQ120479–GQ120499), appear to represent three different taxa within the C. gloeosporioides complex — C. gloeosporioides s. str., C. aotearoa, and C. fructicola. Isolates representative of all three taxa are reportedly pathogenic on Boehmeria (Wang et al. 2010). The genetic relationship of these fungi needs to be confirmed using additional genes.
Colletotrichum camelliae Massee, Bull. Misc. Inform. Kew. 1899: 91. 1899.
Notes: Colletotrichum camelliae was described by Massee (in Willis 1899) from the living leaves of tea (Camellia sinensis) from Sri Lanka. It was placed in synonymy with C. gloeosporioides by von Arx (1957). Although not listed by Hyde et al. (2009), the name is widely used in the trade and semi-popular literature as the causal agent of the brown blight disease of tea (e.g. Sosa de Castro et al. 2001, Muraleedharan & Baby 2007).
We have been unable to sample Colletotrichum isolates from tea with typical brown blight symptoms. There are four GenBank accessions of Colletotrichum from tea, two from China (EU732732, FJ515007), one from Japan (AB218993), and another from Iran (AB548281), referred variously to C. camelliae, C. crassipes and C. gloeosporioides. Although ITS sequences only are available for these geographically widespread isolates, the DNA sequence of the Iranian isolate appears to match C. gloeosporioides s. str., while those from the other three isolates are all very similar to each other. The ITS sequence from these isolates matches that of CBS 232.79, from tea shoots from Java (GenBank JX009429). GAPDH and ITS sequences from CBS 232.79 (GenBank JX009417, JX009429) place this isolate in C. fructicola. Note that CBS 571.88, isolated from tea from China and deposited as Glomerella cingulata, is a Colletotrichum sp. outside C. gloeosporioides s. lat., based on ITS sequences (GenBank JX009424).
We tested the pathogenicity of CBS 232.79 and isolates of G. cingulata “f. sp. camelliae” (see below) using detached tea leaves and found that only the G. cingulata “f. sp. camelliae” isolates were strong pathogens (unpubl. data).
The genetic relationship between the pathogen of ornamental Camellia (here referred to G. cingulata “f. sp. camelliae”), isolates from tea with DNA sequence data in GenBank, and isolates associated with brown blight symptoms of tea remain unresolved. Additional isolates with known pathogenicity, collected from typical brown blight symptoms from the field, are required to determine whether or not there are two distinct pathogens of Camellia, one of tea, the other of ornamental varieties.
Other Colletotrichum species reported from tea include C. “theae-sinensis”, an invalid recombination of Gloeosporium theae-sinensis I. Miyake, proposed by Yamamoto (1960). Moriwaki and Sato (2009) summarised the taxonomic history of this name and transferred G. theae-sinensis to Discula on the basis of DNA sequences. Sphaerella camelliae Cooke and its recombination Laestadia camelliae (Cooke) Berl. & Voglino were listed by von Arx & Müller (1954) as synonyms of Glomerella cingulata. This species is now accepted as Guignardia camelliae (Cooke) E.J. Butler ex Petch and is regarded as the causal agent of copper blight disease of tea (Spaulding 1958).
Thang (2008) placed C. camelliae in synonymy with C. coccodes, presumably on the basis of the Species Fungorum synonymy (www.speciesfungorum.org, website viewed 6 Oct 2010). Thang (2008) questioned the synonymy, noting differences between the descriptions of the two species provided by Massee (in Willis 1899) and Sutton (1980) respectively.
Colletotrichum caricae F. Stevens & J.G. Hall, Z. Pflanzenkrankh., 19: 68. 1909.
Notes: Placed in synonymy with C. gloeosporioides by von Arx (1957), C. caricae was listed as a separate species by Sutton (1992). It was described from fruits and leaves of Ficus carica from the USA (Stevens & Hall 1909) but is poorly understood both morphologically and biologically. Its genetic relationship to and within the C. gloeosporioides species complex, and to other Ficus-associated species such as Colletotrichum ficus Koord. and Glomerella cingulata var. minor (here placed in synonymy with C. fructicola) is unknown.
Glomerella cingulata (Stonem.) Spauld. & H. Schrenk, Science, n.s. 17: 751. 1903.
Basionym: Gnomoniopsis cingulata Stonem., Bot. Gaz. 26: 101. 1898.
= Gloeosporium cingulatum G.F. Atk., Bull. Cornell Univ. Agric. Exp. Sta. 49: 306. 1892. [fide Stoneman 1898]
Notes: Stoneman (1898) described Glomerella cingulata from diseased stems of Ligustrum vulgare from the USA and reported the development of perithecia in cultures initiated from conidia of what she considered its asexual morph, Gloeosporium cingulatum. There are recent reports of anthracnose diseases of Ligustrum (e.g. Alfieri et al. 1984, Vajna & Bagyinka 2002) but the relationship of isolates causing this disease to the C. gloeosporioides complex is not known.
Glomerella cingulata is often linked taxonomically to the anamorph Colletotrichum gloeosporioides, and the name has in the past been applied in an equally broad sense to C. gloeosporioides s. lat. (e.g. Small 1926, von Arx & Müller 1954). However, it is unlikely that the type specimen of G. cingulata represents the same species as C. gloeosporioides s. str. (see notes under C. gloeosporioides). Colletotrichum gloeosporioides s. str. is not known to form perithecia in culture, and there are no isolates of C. gloeosporioides s. str. known to us that are associated with a Glomerella state on diseased stems of Ligustrum, An isolate of C. aotearoa (ICMP 18748) was isolated from Ligustrum lucidum in New Zealand, but it was not associated with a stem lesion and no C. aotearoa isolates were observed forming perithecia.
Glomerella cingulata var. brevispora Wollenw., Z. Parasitenk. (Berlin) 14: 260. 1949.
Notes: Described from fruit rots from Germany, this name has not been used since. No cultures are available and its relationship to and within the C. gloeosporioides complex is not known.
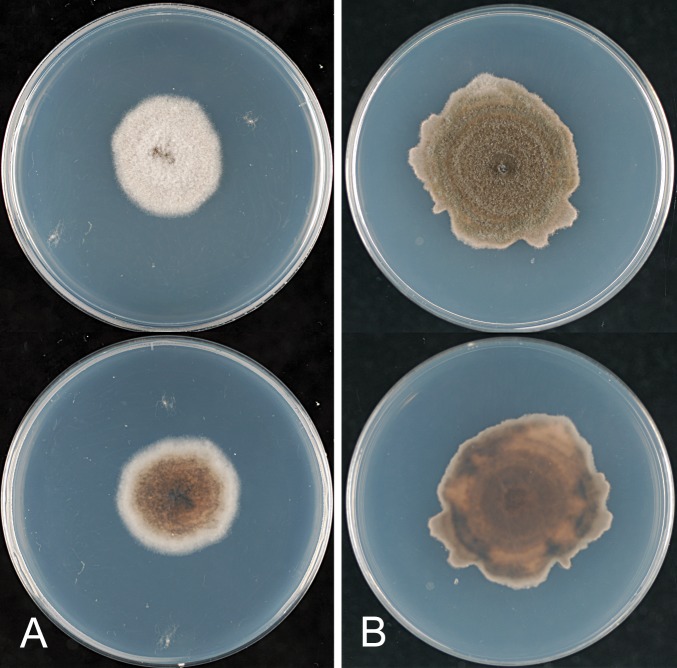
* Glomerella cingulata “f. sp. camelliae” (Dickens & Cook 1989). Figs 18, 19.
Fig. 18.
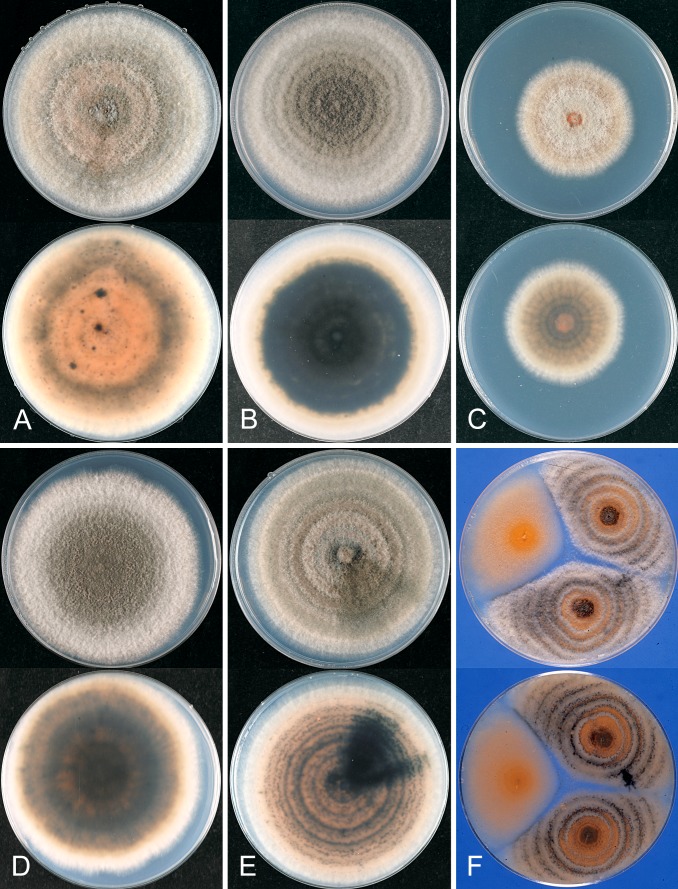
Glomerella cingulata “f. sp. camelliae”. A, C, D. ICMP 10643. B, E. ICMP 10646. A–B. Appressoria. C. Conidiogenous cells. D–E. Conidia. Scale bar A = 20 μm. Scale bar of A applies to A–E.
Fig. 19.
Glomerella cingulata “f. sp. camelliae”. A. ICMP 18542. B. ICMP 10643. C. ICMP 10646. A–C. Cultures on PDA, 10 d growth from single conidia, from above and below.
Notes: Dickens & Cook (1989) proposed the name Glomerella cingulata “f. sp. camelliae” for isolates morphologically typical of C. gloeosporioides s. lat. that were highly pathogenic to leaves and shoots of ornamental Camellia saluenensis hybrids, causing the disease Camellia twig blight. These authors reported the fungus from plants imported into the UK from New Zealand and noted that a similar disease had been reported from plants grown in the UK, USA, Australia, France, and Italy. The disease has been reported from Camellia japonica, C. reticulata, and C. sasanqua. Although isolated in the UK from plants imported from New Zealand, this pathogen has not yet been found on Camellia plants growing in New Zealand.
We have sequenced authentic isolates cited by Dickens & Cook (1989) as well as isolates pathogenic to Camellia saluenensis collected from the USA. They are similar to each other genetically as well as biologically and morphologically. ITS sequences alone distinguish G. cingulata “f. sp. camelliae” from all other taxa in the C. gloeosporioides complex, and there is good genetic evidence to consider these isolates to be representative of a distinct species within the C. kahawae clade. A new species is not proposed here because the relationship between the G. cingulata “f. sp. camelliae” isolates and C. camelliae, the fungus causing brown blight of tea, remains uncertain.
Dickens & Cook (1989) also reported two C. acutatum strains from ornamental Camellia species that were avirulent in tests with detached Camellia cv. Donation leaves. Strain IMI 351261, deposited 1992 in IMI by R. Cook, is likely to be one of them. This strain was confirmed as belonging to the C. acutatum species complex and identified as C. lupini, which causes lupin anthracnose and is occasionally found on other hosts (Damm et al. 2012a, this issue). Another strain from Camellia reticulata from China belongs to C. fioriniae, also a species in the C. acutatum complex, while a strain from New Zealand (ICMP 10338) is C. boninense s. str. (Damm et al. 2012a, b, this issue).
See notes under C. camelliae.
Specimens examined: UK, plants imported from New Zealand, on Camellia × williamsii, coll. Dickens & Cook 82/437, 1982 (authentic culture of Glomerella cingulata “f. sp. camelliae” – ICMP 10643; dried culture PDD 56488). USA, Mississippi, on Camellia sasanqua twig blight, coll. W.E. Copes CG02g, May 2002 (ICMP 18542); South Carolina, on Camellia sp., coll. G. Laundon 20369, 1 Jan. 1982 (ICMP 10646).
Glomerella cingulata var. crassispora Wollenw., Z. Parasitenk. (Berlin) 14: 260. 1949.
Notes: Described from Coffea arabica from a glasshouse in Germany, this name has not been used since. No cultures are available and its relationship to and within the C. gloeosporioides complex is not known.
Glomerella cingulata “f. sp. manihotis” (Chevaugeon 1956)
Notes: See notes under Colletotrichum manihotis.
Glomerella cingulata var. minor Wollenw., Z. Parasitenk. (Berlin) 14: 261. 1949.
= Gloeosporium elasticae Cooke & Massee, Grevillea 18: 74. 1890. [fide Wollenweber & Hochapfel 1949]
Notes: Placed here in synonymy with C. fructicola.
Glomerella cingulata var. minor was described from Ficus from Germany, but Wollenweber & Hochapfel (1949) noted that the same fungus occurred also on other hosts in Europe, Africa, and America, including Malus and Coffea. Genetically the ex-holotype culture of G. cingulata var. minor (CBS 238.49) matches the type specimen of C. fructicola, although the culture itself appears to be stale, with slow growth and an irregularly scalloped margin (see images under C. fructicola). Wollenweber & Hochapfel (1949) used the name Gloeosporium elasticae Cooke & Massee for the conidial state of G. cingulata var. minor, the type specimens for both names being from Ficus.
See also notes under C. queenslandicum.
Specimen examined: Germany, Berlin-Dahlem, from Ficus edulis leaf spot, May 1936 (ex-holotype culture of G. cingulata var. minor – CBS 238.49 = ICMP 17921).
Glomerella cingulata var. migrans Wollenw., Z. Parasitenk. (Berlin) 14: 262. 1949.
Notes: Placed here in synonymy with C. kahawae subsp. ciggaro, see notes under this species.
Specimen examined: Germany, Berlin-Dahlem, on stem of Hypericum perforatum, Jun. 1937 (ex-holotype culture of Glomerella cingulata var. migrans – CBS 237.49 = ICMP 17922).
Glomerella cingulata “var. orbiculare” Jenkins & Winstead, Phytopathology 52: 15. 1962.
Notes: Listed in Index Fungorum, this name was mentioned in an abstract, but is invalid (no Latin description) and never formally published. It was being used to refer to the teleomorph of Colletotrichum orbiculare, not part of the C. gloeosporioides complex (Cannon et al. 2012, this issue). Glomerella lagenaria (Pass.) F. Stevens, a recombination of the anamorphic name Fusarium lagenarium Pass., has also been used to refer to this teleomorph. Correll et al. (1993) comment on the pathogenicity of cucurbit-associated strains that form a Glomerella state in culture, suggesting a degree of confusion around the application of these names.
Glomerella cingulata “f. sp. phaseoli” (Kimati & Galli 1970).
Notes: Both G. cingulata “f. sp. phaseoli” (e.g. Castro et al. 2006) and Glomerella lindemuthiana (e.g. Rodríguez-Guerra et al. 2005, as G. lindemuthianum) have been used for the teleomorph of Colletotrichum lindemuthianum in the recent literature, the two names placed in synonymy by Sutton (1992). This fungus is not part of the C. gloeosporioides complex (Cannon et al. 2012, this issue).
Glomerella cingulata var. sorghicola Saccas, Agron. Trop. (Maracay). 9: 171. 1954.
Notes: Not a member of the C. gloeosporioides complex. Sutton (1992) suggested using this name to refer to the teleomorph of Colletotrichum sublineola, although Crouch et al. (2006) note that C. sublineola has no known teleomorph.
* Colletotrichum clidemiae B. Weir & P.R. Johnst. sp. nov. MycoBank MB563592. Figs 20, 21.
Fig. 20.
Colletotrichum clidemiae. A, B, E. ICMP 18658 – ex-holotype culture. C, D. ICMP 18706. A, D. Appressoria. B, C. Asci and ascospores. E. Conidia. Scale bar C = 20 μm. Scale bar of C applies to A–E.
Fig. 21.
Colletotrichum clidemiae. A. ICMP 18658 – ex-holotype culture. ICMP 18706. Cultures on PDA, 10 d growth from single conidia, from above and below.
= Colletotrichum gloeosporioides “f. sp. clidemiae” (Trujillo et al. 1986).
Etymology: Based on the host reportedly susceptible to this species.
Holotype: USA, Hawai’i, Aiea, on Clidemia hirta leaf spot, coll. S.A. Ferreira & K. Pitz, 14 May 2010, PDD 101997; ex-type culture ICMP 18658.
Colonies grown from single conidia on Difco PDA 25 mm diam after 10 d, aerial mycelium grey, cottony, sparse, surface of colony with numerous small, dark-based acervuli with deep orange conidial ooze and scattered setae, in reverse more or less colourless except for the acervuli and masses of conidial ooze showing through. After 18 d numerous globose, pale walled protoperithecia developing near centre of colony. Conidia (16–)18–20(–26.5) × (4.5–)5.5–6 μm (av. 19.3 × 5.5 μm, n = 48), broad-cylindric, ends broadly rounded, longer conidia sometimes tapering slightly towards the base. Appressoria variable in shape, some simple, subglobose, but often with a small number of broad, irregular lobes. Perithecia mature after about 21 d, dark-walled, about 200–250 μm diam with short ostiolar neck, perithecial wall of 3–4 layers of angular cells 10–15 μm diam with walls thin, pale brown to brown. Asci 8-spored 60–67 × 10–14 μm. Ascospores (14–)15.5–19(–21.5) × 4.5–5.5(–6.5) μm (av. 17.2 × 5.0 μm, n = 46), oblong-elliptic, tapering to rounded ends, widest point toward one end, in side view flat on one side, rarely curved and if so, then slightly.
Geographic distribution and host range: First reported from Clidemia, native to Panama, and subsequently introduced to Hawai’i as a pathogen of that host. Genetically matching isolates occur on native Vitis and Quercus spp. in Florida (see notes below).
Genetic identification: ITS sequences do not separate C. clidemiae from C. aotearoa. The two species are best distinguished using ACT, GAPDH, or GS.
Notes: Isolates referred to C. gloeosporioides “f. sp. clidemiae” by Trujillo et al. (1986) were highly pathogenic to Clidemia, but not to the other species of Melastomataceae tested. No voucher cultures of the original isolates collected from Panama were kept, but recent specimens isolated from naturalised Clidemia hirta plants in Hawai’i with typical disease symptoms are genetically uniform and distinct within the Kahawae clade. Phylogenetic, biological, and morphological evidence support this fungus being described as a new species within the C. gloeosporioides complex.
A fungus isolated from a Vitis sp. in Florida and referred to as “Glomerella cingulata native host” by MacKenzie et al. (2007), is genetically close to our isolates from Clidemia and is here referred to the same species. Data in MacKenzie et al. (2007) shows the same fungus occurs on both Vitis and Quercus in Florida. Micro-morphologically the isolates from Clidemia and from Vitis that we examined are similar with respect to the size and shape of appressoria, conidia, and ascospores. They are distinct in cultural appearance, the cultures of the Vitis-associated fungus having aerial mycelium darker and more dense, and a faster growth rate. Similar variation in cultural appearance is present in several of the phylogenetically defined species that we recognise. Whether or not the Clidemia-associated isolates are biologically distinct from the Vitis- and Quercus-associated isolates from Florida requires pathogenicity tests to determine.
Other specimens examined: USA, Florida, Sarasota, on Vitis sp. leaf, coll. S. MacKenzie SS-Grape-12, 2002 (ICMP 18706); Hawai’i, Aiea, on Clidemia hirta leaf spot, coll. S.A. Ferreira & K. Pitz, 14 May 2010 (ICMP 18659, ICMP 18660, ICMP 18661, ICMP 18662, ICMP 18663).
Colletotrichum coffeanum F. Noak, Z. Pflanzenkrankh. 11: 202. 1901.
Notes: Waller et al. (1993) discussed the use of the names Colletotrichum coffeanum and Gloeosporium coffeanum Delacr. and the geographic and biological differences between these species and the pathogen of coffee berries, C. kahawae. Both C. coffeanum and G. coffeanum were described from leaves of coffee, the two species distinguished by Noak (1901) by the presence or absence of setae in the acervuli. There is a wide range of C. gloeosporioides-like species on coffee plants (see Waller et al. 1993 and notes under C. kahawae) and the relationships of C. coffeanum and G. coffeanum within the C. gloeosporioides species complex remain uncertain.
Colletotrichum cordylines Pollacci, Atti Ist. Bot. Univ. Pavia, Serie 2, 5: 44. 1899.
Notes: Described from leaves of Cordyline indivisa from a botanical garden in Italy, the genetic and biological status of this species is not known. Two Cordyline-associated species are accepted in this study, C. cordylinicola from Thailand and the newly described C. ti from New Zealand. The original description of C. cordylines is brief (Pollacci 1899) but it specifically mentions setae more than 100 μm long. Colletotrichum cordylinicola is described as lacking setae (Phoulivong et al. 2011) and in C. ti they are rare and when present much less than 100 μm long. The phylogenetic significance of this apparent difference and confirmation that these names represent different fungi requires DNA sequences to be generated from type material of C. cordylines.
* Colletotrichum cordylinicola Phoulivong, L. Cai & K.D. Hyde, Mycotaxon 114: 251. 2011 [“2010”]. Fig. 22.
Fig. 22.
Colletotrichum cordylinicola. ICMP 18579 (ex MFLUCC 090551 – ex-holotype culture). A. Cultures on PDA, 10 d growth from single conidia, from above and below.
Phoulivong et al. (2011) provide a description.
Geographic distribution and host range: Known only from Cordyline from Thailand and Eugenia from Laos.
Genetic identification: ITS sequences separate C. cordylinicola from all other species.
Notes: Phoulivong et al. (2011) report C. cordylinicola from Cordyline (Agavaceae) and Eugenia (Myrtaceae). They noted that the isolate from Eugenia was not pathogenic to Cordyline and vice versa, and they also showed that the specimens from the two hosts are genetically somewhat distinct, although forming a sister relationship amongst the taxa included in their analysis. The calmodulin gene tree generated from our sequence data together with the sequences provided by Phoulivong et al. (2011) (GenBank accession HM470236) supports placing the isolates from Eugenia and from Cordyline in the same species (unpubl. data).
Colletotrichum cordylinicola is genetically distinct from a species associated with Cordyline leaf spots from New Zealand, described here as C. ti. See also notes under C. cordylines.
Specimen examined: Thailand, Chiang Mai, Sam Sai District, Maejo Village, on Cordyline fruticosa, coll. S. Phoulivong, 15 Mar. 2009 (ex-holotype culture – MFLUCC 090551 = ICMP 18579). Note that the ex-holotype culture was mistakenly cited as MFUCC 090551 by Phoulivong et al. (2011).
Colletotrichum crassipes (Speg.) Arx, Verh. Kon. Ned. Akad. Wetensch., Afd. Natuurk., Sect. 2, 51(3): 77. 1957. Basionym: Gloeosporium crassipes Speg., Rivista Vitic. Enol. 2: 405. 1878.
Notes: Several isolates identified as Colletotrichum crassipes that have sequences accessioned to GenBank belong in C. gloeosporioides s. lat. GenBank accessions identified as C. crassipes that have a publically available culture include C. kahawae subsp. ciggaro (STE-U 5302 = CBS 112988 – AY376529, AY376577, FN557348, FN557538, and FN599821; STE-U 4445 = CBS 112984 – AY376530, AY376578, – FN557347, FN557537, and FN599820), along with several other species outside of the C. gloeosporioides complex (CBS 169.59 = IMI 309371 – AJ536230, FN557344, and FN599817; CBS 159.75 – FN557345 and FN599818; CBS 109355 – FN557346 and FN599819). Those with no isolates in a public collection include C. kahawae subsp. ciggaro (CORCS3 cited in Yang et al. (2011), HM584410, HM582002, HM585412), C. fructicola (strain 080912009 Jining, unpubl. data, FJ515007), and a possibly undescribed species within the Kahawae clade (strain SYJM02, unpubl. data, JF923835). Originally described from the berries of Vitis vinifera from Italy (Spegazzini 1878), the identity of C. crassipes remains unresolved. There is confusion regarding its morphology. Von Arx (1970) uses the name C. crassipes for fungi in which setae are rare, conidia are 22–34 × 6–8 μm (more or less matching the original description), and the lobed appressoria are distinctively globose in shape. Sutton (1980) uses a different morphological concept – setae common (according to Sutton these are rare in the otherwise morphologically similar C. musae), conidia 10–15 × 4.5–6.5 μm (Sutton’s concept of C. gloeosporioides is characterised by narrower conidia), and the appressoria deeply lobed. The conidial width cited for C. gloeosporioides by Sutton (1980), 3–4.5 μm, is narrower than we have found for all the taxa we accept within C. gloeosporioides s. lat., whereas his C. crassipes measurement of 4.5–6.5 μm matches many of the taxa we recognise. Several of these taxa also have deeply lobed appressoria.
Colletotrichum dracaenae Allesch., Rabenhorst’s Kryptogamen-Flora von Deutschland, Oesterreich und der Schweiz, Ed. 2, 1(7): 560. 1902.
Notes: Farr et al. (2006) examined the type specimen of this species and concluded it was a member of C. gloeosporioides s. lat., based on conidial size and shape. Genetic data is not available to confirm this. See also discussion under C. petchii in Damm et al. (2012b, this issue)
Colletotrichum fragariae A.N. Brooks, Phytopathology 21: 113. 1931.
Notes: Placed here in synonymy with C. theobromicola. See notes and additional specimens examined under C. theobromicola.
The name C. fragariae was originally applied to isolates associated with a disease of strawberry (Fragaria × ananassa) runners (stolons) and petioles in Florida (Brooks 1931). Although the name was placed in synonymy with C. gloeosporioides by von Arx (1957), it has continued to be used in the literature for strawberry-associated Colletotrichum isolates. It was accepted as distinct by Sutton (1992), although he noted confusion surrounding application of the name. Designation of one of Brook’s cultures (CBS 142.31 = IMI 346325) as the epitype of C. fragariae by Buddie et al. (1999) has allowed a modern, genetic basis for this name to be fixed. The ex-epitype culture of C. fragariae sits in a strongly supported clade containing isolates from a wide range of hosts from many parts of the world, including the ex-epitype culture of C. theobromicola, an earlier name for C. fragariae in the sense that we accept these species in this paper.
There are several species from the C. gloeosporioides complex which inhabit diseased strawberry plants, and as shown by MacKenzie et al. (2007, 2008) isolates that genetically match the epitype of C. fragariae have a wide host range. Despite its name MacKenzie et al. (2007, 2008) regarded this fungus as simply one of a group of several species sometimes found on strawberry. Our study confirms that members of the C. fragariae/theobromicola clade occur throughout the world on a wide range of hosts. Within the diversity of the C. fragariae/theobromicola clade, there is a subclade consisting of the C. fragariae epitype and two contemporary ex-strawberry isolates from the USA (Fig. 1), further work will be needed to establish if the strawberry stolon disease is restricted to this subclade. Despite regular surveys this disease has not been found on strawberries in New Zealand.
Xie et al. (2010b) provides a good example of the confusion that continues to surround the application of Colletotrichum names to isolates from strawberry. These authors noted that putative C. gloeosporioides and C. fragariae isolates were difficult to distinguish using ITS sequences, the only sequences that they generated. Xie et al. (2010b) found 4 groups of isolates, each with a slightly different ITS sequence, two of those groups they considered to be C. fragariae and two to be C. gloeosporioides. To classify their isolates as either C. fragariae of C. gloeosporioides they used a restriction enzyme method based on Martinez-Culebras et al. (2000). Incorporating their ITS sequences into our ITS alignment, one of their groups genetically matches C. tropicale, one matches C. gloeosporioides s. str., one matches C. fructicola, and one matches C. siamense. These relationships are based on ITS sequences only — the genetic differences between some of these species are small and are indicative only of possible relationships. However, it is clear that none of the Xie et al. (2010b) sequences match those of the epitype of C. fragariae. There are also several species within the C. acutatum species complex associated with Fragaria (Damm et al. 2012, this issue).
Specimen examined: USA, Florida, on Fragaria × ananassa, coll. A.N. Brooks, 1931 (ex-epitype culture – CBS 142.31 = ICMP 17927).
* Colletotrichum fructicola Prihastuti, L. Cai & K.D. Hyde, Fungal Diversity 39: 158. 2009. Fig. 23.
Fig. 23.
Colletotrichum fructicola. A. ICMP 12568. B. ICMP 18615. C. ICMP 18581 (ex MFLU 090228 – ex-holotype culture of C. fructicola). D. ICMP 18610. E. ICMP 18646 (ex CBS 125379 – ex-holotype culture of C. ignotum). F. ICMP 17921 (ex CBS 238.49 – ex-holotype culture of G. cingulata var. minor). A–F. Cultures on PDA, 10 d growth from single conidia, from above and below.
= Colletotrichum ignotum E.I. Rojas, S. A. Rehner & Samuels, Mycologia 102: 1331. 2010.
= Glomerella cingulata var. minor Wollenw., Z. Parasitenk. (Berlin) 14: 261. 1949.
Prihastuti et al. (2009) and Rojas et al. (2010) provide descriptions.
Geographic distribution and host range: Originally reported from coffee berries from Thailand (as C. fructicola) and as a leaf endophyte from several plants in Central America (as C. ignotum), isolates that we accept as C. fructicola are biologically and geographically diverse. Known from Coffea from Thailand, Pyrus pyrifolia from Japan, Limonium from Israel, Malus domestica and Fragaria × ananassa from the USA, Persea americana from Australia, Ficus from Germany, Malus domestica from Brazil, Dioscorea from Nigeria, and Theobroma and Tetragastris from Panama.
Genetic identification: ITS sequences do not separate C. fructicola from C. aeschynomenes and some C. siamense isolates. These taxa are best distinguished using GS or SOD2.
Notes: Rojas et al. (2010) noted the occurrence of two distinct haplotype subgroups (A4-3 and A5-4) within their concept of C. ignotum. Our genetic analyses resolve the two clades representative of these two subgroups. However, together they are monophyletic within the Musae clade of the C. gloeosporioides complex, and we retain them here as a single species. Both clades include isolates from a wide range of hosts from many countries, and both are similar in morphology and cultural appearance. The types of both C. fructicola and C. ignotum are in the same haplotype subgroup.
The C. fructicola protologue designates the holotype as MFLU 090228, but the culture derived from holotype as “BCC” with no specimen number. The ex-holotype culture is listed as BDP-I16 in Table 1 of Prihastuti et al. (2009) but this number is not mentioned in the description. Culture BDP-I16 was obtained from the authors (Prihastuti et al. 2009) for this study and deposited as ICMP 18581.
See also notes under G. cingulata var. minor.
Specimens examined: Australia, Queensland, Bli-Bli, on Persea americana fruit rot, coll. L. Coates 24154 (ICMP 12568). Brazil, Rio Grande do Sul State, on Malus domestica leaf, coll. T. Sutton BR 8 2001, Jan. 2001 (ICMP 17787); Santa Catarina State, on Malus domestica leaf, coll. T. Sutton BR 21 2001, Jan. 2001 (ICMP 17788).
Canada, Ontario, on Fragaria × ananassa, Jan. 1991 (IMI 345051 = ICMP 17819). Germany, Berlin-Dahlem Botanical Garden, on Ficus edulis leaf spot, (ex-holotype culture of Glomerella cingulata var. minor – CBS 238.49 = ICMP 17921). Indonesia, Java, Bandung, Pangheotan Estate, on Camellia sinensis shoots, coll. H. Semangun, Apr. 1979 (CBS 232.79 = ICMP 18656). Israel, on Limonium sinuatum leaf lesion, coll. S. Freeman L32 (cited in Moriwaki et al. 2006) (ICMP 18613); on Limonium sp. leaf lesion, coll. S. Freeman L50 (cited in Maymon et al. 2006) (ICMP 18698); on Limonium sp. leaf lesion, coll. S. Freeman Cg2 (cited in Maymon et al. 2006) (ICMP 18667); on Limonium sinuatum, coll. S. Freeman L11 (cited in Maymon et al. 2006) (ICMP 18615). Japan, Saga, on Pyrus pyrifolia, coll. H. Ishii sA02-5-1 (cited in Chung et al. 2006) (ICMP 18610). Nigeria, Ibadan, on Dioscorea alata leaf spot, M. Abang Cg13 (cited in Abang et al. 2002) (ICMP 18125); Ilesha, Dioscorea rotundata leaf spots, coll. M. Abang Cg22 (cited in Abang et al. 2002) (ICMP 18120). Panama, Barro Colorado Monument, Tetragastris panamensis leaf endophyte, coll. E.I. Rojas E886, 1 Jun. 2004 (ex-holotype culture of C. ignotum – CBS 125397 = ICMP 18646); Theobroma cacao leaf endophyte, coll. E. Rojas E183 (CBS 125395 = ICMP 18645). Thailand, Chiang Mai, Pa Daeng Village, on Coffea arabica berry, coll. H. Prihastuti BPD-I16, 12 Dec. 2007 (ex-holotype culture of C. fructicola, from specimen MFLU 090228 – ICMP 18581 = CBS 130416). USA, on Fragaria × ananassa crown, F. Louws 9, (ICMP 18727); Florida, on Fragaria × ananassa, coll. F.A. Ueckes FAU552 (CBS 120005 = BPI 747977 = ICMP 18609); North Carolina, Lincoln County, on Malus domestica fruit, coll. T. Sutton CROTTS 13 2001, Jan. 2001 (ICMP 17789).
* Colletotrichum gloeosporioides (Penz.) Penz. & Sacc., Atti Reale Ist. Veneto Sci. Lett. Arti., Serie 6, 2: 670. 1884. Fig. 24.
Fig. 24.
Colletotrichum gloeosporioides. A. ICMP 17821 (ex IMI 356878 – ex-epitype culture). A. Cultures on PDA, 10 d growth from single conidia, from above and below.
Basionym: Vermicularia gloeosporioides Penz., Michelia 2: 450. 1882.
= Gloeosporium pedemontanum Pupillo, Ann. Sperim. Agrar. n.s. 6: 57. 1952.
Cannon et al. (2008) provide a description of the species.
Geographic distribution and host range: Most isolates of C. gloeosporioides are associated with Citrus, and in many parts of the world this fungus is common on Citrus, but it also occurs on other hosts including Ficus, Mangifera, Pueraria, and Vitis. The isolate reported as a pathogen of paper mulberry (Broussonetia papyrifera) by Yan et al. (2011) matches C. gloeosporioides s. str. genetically.
Genetic identification: ITS separates C. gloeosporioides from all other species.
Notes: The name Colletotrichum gloeosporioides is currently in common use in two senses, one a genetically and biologically broad sense more or less following von Arx (1957, 1970) and Sutton (1992), including the whole species complex, the other a strict sense, encompassing only those specimens genetically matching the epitype selected for this name by Cannon et al. (2008). Depending on the context, use of the name in either sense can be useful. When used in a broad sense in this paper, it is referred to as the C. gloeosporioides species complex or C. gloeosporioides s. lat.
Colletotrichum gloeosporioides is often linked taxonomically to the teleomorph Glomerella cingulata, see notes under G. cingulata.
Specimens examined: Australia, New South Wales, Tamworth, on Carya illinoinensis (DAR 76936; ICMP 18738). Italy, Calabria, on Citrus sinensis, (ex-epitype culture of C. gloeosporioides – IMI 356878 = CBS 112999 = ICMP 17821); on Citrus limon juice, coll. G. Goidánich, 1951 (ex-holotype culture of Gloeosporium pedemontanum – CBS 273.51 = ICMP 19121). New Zealand, Auckland, Sandringham, on Citrus sp. fruit, coll. P.R. Johnston C1014.6, 2 May 1988 (ICMP 12939); Auckland, Sandringham, on Ficus sp. fruit, coll. P.R. Johnston C945.2, 9 May 1988 (ICMP 12066); Auckland, on Citrus sp. fruit, coll. G. Caroll, Feb 2010 (ICMP 18730); Northland, Kerikeri, Kapiro Rd, on Citrus sinensis fruit, coll. P.R. Johnston C1009.2, 10 Aug. 1988 (ICMP 12938). South Africa, on Mangifera indica, coll. L. Korsten Cg68 (ICMP 18694). USA, Georgia, on Pueraria lobata (AR2799 = CBS 119204 = BPI 871837 = ICMP 18678); Florida, on Citrus sp. leaf lesion, coll. N. Peres SRL-FTP-9 (ICMP 18695); Florida, on Vitis vinifera leaf lesion, coll. N. Peres LAGrape8 (ICMP 18697).
Colletotrichum gloeosporioides “f. sp. aeschynomenes” (Daniel et al. 1973, as aeschynomene).
Notes: See Colletotrichum aeschynomenes.
Colletotrichum gloeosporioides “f. alatae” R.D. Singh, Prasad & R.L. Mathur, Indian Phytopathol. 19: 69. 1966. [nom. inval., no Latin description, no type designated]
Notes: See Colletotrichum alatae.
Colletotrichum gloeosporioides var. aleuritis Saccas & Drouillon [as “aleuritidis”], Agron. Trop. (Nogent-sur-Marne) 6: 249. 1951.
≡ Glomerella cingulata var. aleuritis Saccas & Drouillon [as “aleuritidis”], Agron. Trop. (Nogent-sur-Marne) 6: 251. 1951.
Notes: Originally described from Aleurites fordii and A. montaba from French Equatorial Africa, these names have not been used since being described and the genetic relationship of this fungus to and within the C. gloeosporioides species complex is unknown. Although the original publications have not been seen, both names were tagged as invalid in the Index of Fungi 2: 53, 57 (1952).
Colletotrichum gloeosporioides “f. sp. clidemiae” (Trujillo et al. 1986).
Notes: See Colletotrichum clidemiae.
Colletotrichum gloeosporioides “f. sp. cucurbitae” (Menten et al. 1980).
Notes: First described from cucumber, this fungus is widely regarded as a synonym of C. orbiculare in the plant pathology literature (e.g. Snowdon 1991, da Silva et al. 2011).
Colletotrichum gloeosporioides “f. sp. cuscutae” (Zhang 1985).
Notes: A strain identified by this name was developed as a mycoherbicide against dodder (Cuscuta chinensis in China (Zhang 1985). This strain referred to as “Lu Bao No.1” is apparently included in the study of Guerber et al. (2003) as strain 783 and belongs to the C. acutatum species complex. Other strains from dodder in the USA included in the same study were revealed to be C. fioriniae, while a strain from Dominica was found to represent a new species, both belonging to the C. acutatum species complex as well (Damm et al. 2012, this issue).
Colletotrichum gloeosporioides var. gomphrenae Perera, Revista Fac. Agron. Univ. Nac. La Plata 41: 12. 1965.
Notes: Originally described from Gomphrena globosa, the name has not been used since it was described and its genetic relationship to and within the C. gloeosporioides species complex is unknown.
Colletotrichum gloeosporioides var. hederae Pass., Atti Reale Accad. Italia, Rendiconti., Serie 4, 6: 469. 1889.
Notes: The original description of this Hedera-inhabiting species, with fusiform, straight to curved conidia suggests that it is a synonym of the Hedera pathogen C. trichellum.
Colletotrichum gloeosporioides f. heveae (Petch) Saccas, Agron. Trop. (Nogent-sur-Marne) 14: 430. 1959.
Basionym: Colletotrichum heveae Petch, Ann. Roy. Bot. Gard. Peradeniya 3(1): 8. 1906.
Notes: Originally described from the leaves of seedlings of Hevea brasiliensis from Sri Lanka, this fungus was described with very broad conidia, 18–24 × 7.5–8 μm. Carpenter & Stevenson (1954) considered this, and several other Colletotrichum, Gloeosporium and Glomerella species described from rubber, to be synonyms of C. gloeosporioides. The genetic relationship of these species to and within the C. gloeosporioides species complex is unknown. See also notes in Damm et al. (2012b, this issue) under Colletotrichum annelatum.
Colletotrichum gloeosporioides “f. sp. hyperici” (Harris 1993).
Notes: This name was first used by Harris (1993) for strains of C. gloeosporioides pathogenic to Hypericum perforatum. Earlier studies by Hildebrand & Jensen (1991) had found the Hypericum pathogen to be pathogenic also on several other plants. The genetic relationship of the Hypericum pathogen to and within the C. gloeosporioides species complex is unknown. Note that the ex-holotype culture of G. cingulata var. migrans, a variety here placed in synonymy with C. kahawae subsp. ciggaro, was also isolated from Hypericum.
Colletotrichum gloeosporioides “f. sp. jussiaeae” (Boyette et al. 1979).
Notes: Strains identified as C. gloeosporioides “f. sp. jussiaeae” are highly pathogenic, specialised pathogens of Jussiaea decurrens (Boyette et al. 1979). The genetic relationship of this taxon to and within the C. gloeosporioides species complex, or to Colletotrichum jussiaeae Earle, is unknown. Isolates pathogenic to Jussiaea have a similar conidial germination self-inhibitor profile to another isolate identified as C. fragariae (Tsurushima et al. 1995). The authentic isolate of C. gloeosporioides “f. sp. jussiaeae” deposited as ATCC 52634, is not included in this study.
Colletotrichum gloeosporioides “f. sp. malvae” (Makowski & Mortensen 1989).
Notes: Strains identified as C. gloeosporioides “f. sp. malvae” were registered as a bioherbicide against round leafed mallow in Canada (Makowski & Mortensen 1989). The fungus was subsequently recognised as belonging to the C. orbiculare species complex (Bailey et al. 1996).
Colletotrichum gloeosporioides “f. sp. manihotis” (Chevaugeon 1956).
Notes: See Colletotrichum manihotis.
Colletotrichum gloeosporioides f. melongenae Fournet, Ann. Mus. Civico Storia Nat. Genova 5: 13. 1973.
Notes: In addition to C. gloeosporioides f. melongenae, the names C. gloeosporioides “f. sp. melongenae”, C. melongenae Av.-Saccá 1917, and C. melongenae Lobik 1928 have been used to refer to fungi associated with anthracnose diseases of Solanum melongena (e.g. Sherf & McNab 1986, Kaan 1973). Other names used for isolates from the same host have included Gloeosporium melongenae Ellis & Halst. 1891 and G. melongenae Sacc. 1916. The genetic relationships of these eggplant-associated taxa to and within the C. gloeosporioides species complex remain unknown. Solanum melongena associated species are known also from the C. boninense species complex (Damm et al. 2012b, this issue).
Colletotrichum gloeosporioides “f. sp. miconiae” (Killgore et al. 1999).
Notes: Killgore et al. (1999) reported that the isolates they recognised as C. gloeosporioides “f. sp. miconiae” were highly specialised pathogens of Miconia calvescens, unable to infect the closely related Clidemia hirta. The original voucher cultures are no longer available (pers. comm., Robert Barreto). Recently collected isolates from Miconia from the type locality in Brazil have proved to be genetically diverse across the C. gloeosporioides species complex, with isolates in both the Kahawae and Musae clades (unpubl. data). For now the genetic position of this pathogen remains unresolved.
Colletotrichum gloeosporioides var. minus Simmonds, Queensland J. Agric. Anim. Sci. 25: 178A. 1968.
Notes: See Colletotrichum queenslandicum.
Colletotrichum gloeosporioides var. nectrioidea Gonz. Frag., Bol. Soc. Brot., 2: 52. 1924.
Notes: Originally described from Citrus aurantium from Portugal, the name has not been used since it was described and its genetic relationship to and within the C. gloeosporioides species complex is unknown.
Colletotrichum gloeosporioides “f. sp. ortheziidae” (Marcelino et al. 2008).
Notes: Marcelino et al. (2008) clearly show that the Orthezia praelonga pathogen belongs in the C. acutatum species complex, despite referring to the fungus only as C. gloeosporioides “f. sp. ortheziidae”. See also notes under C. nymphaeae in Damm et al. (2012a, this issue).
Colletotrichum gloeosporioides “f. sp. pilosae” (Singh 1974).
Notes: First described from leaves of Bidens pilosa, this name has not been used since it was described and its genetic relationship to and within the C. gloeosporioides species complex is unknown.
Colletotrichum gloeosporioides f. stylosanthis Munaut, Mycol. Res. 106: 591. 2002.
Notes: Placed here in synonymy with C. theobromicola; see notes under C. theobromicola.
Irwin & Cameron (1978) and Munaut et al. (2002) described different diseases of Stylosanthes associated with Type A and Type B isolates of C. gloeosporioides f. stylosanthis, the two groups of isolates distinguished morphologically by growth rate in culture and by conidial morphology. Compared with Type A, the Type B isolates had a slower growth rate on PDA, and conidia more variable in size and shape (Irwin & Cameron 1978). They were also distinguished genetically using RFLP and similar methods (e.g. Munaut et al. 1998, 2002). Munaut et al. (2002) used ITS1 sequences to show the C. gloeosporioides f. stylosanthis to be related to an isolate they identified as C. fragariae. We regard C. fragariae to be a synonym of C. theobromicola, with putatively authentic Type A (HM335, C. gloeosporioides f. stylosanthis “f. sp. guianensis”) and Type B (HM 336, C. gloeosporioides f. stylosanthis “f. sp. stylosanthis”) isolates both also belonging to this species. From the ITS1 sequence data available, isolates regarded as typical of Type A (RAPD cluster I) and of Type B (RAPD cluster II) by Munaut et al. (1998) all belong in C. theobromicola in the sense that we are using the name; their RAPD cluster III isolate could be C. tropicale, and their RAPD cluster IV isolates are probably C. fructicola.
The cultures of C. gloeosporioides f. stylosanthis that we used were originally studied by Irwin & Cameron (1978), and selected as the “types” of “f. sp. guianensis” and “f. sp. stylosanthis” by Munaut et al. (2002). Both isolates have a ‘stale’ growth form, no longer forming conidia in culture and with aerial mycelium closely appressed to the agar surface, resulting in an almost slimy colony surface. Both isolates had a slow growth rate, similar to that reported for Type B isolates by Irwin & Cameron (1978). Genetically both isolates were identical for all the genes we sequenced. This identity should be checked against additional isolates, especially some matching Type A sensu Irwin & Cameron (1978) with respect to both pathogenicity and growth form.
Sherriff et al. (1994), using ITS2 and partial 28S rDNA sequences, found isolates they considered to represent C. gloeosporioides f. stylosanthis Type A and Type B respectively to be genetically distinct. However, their ITS2 sequences show that the putative Type B isolate in their study was in fact a member of the C. boninense species complex.
Specimens examined: Australia, Queensland, Townsville, on Stylosanthes viscosa, coll. J.A.G. Irwin 21365 (HM335), 1976 (ex-holotype culture of C. gloeosporioides f. stylosanthis – MUCL 42294 = ICMP 17957 = CBS 124251); Samford, on Stylosanthes guianensis, coll. J.A.G. Irwin 21398 (HM336), 1979 (MUCL 42295 = ICMP 17958 = CBS 124250).
Colletotrichum gloeosporioides f. stylosanthis “f. sp. guianensis” (Munaut et al. 2002)
≡ Colletotrichum gloeosporioides “f. sp. guianensis” (Vinijsanum et al. 1987).
Notes: See notes and specimens examined under C. gloeosporioides f. stylosanthis.
Colletotrichum gloeosporioides f. stylosanthis “f. sp. stylosanthis” (Munaut et al. 2002).
Notes: See notes and specimens examined under C. gloeosporioides f. stylosanthis.
Colletotrichum gloeosporioides “f. sp. uredinicola” (Singh 1975).
Notes: Described from uredinia and telia of Ravenelia sessilis on pods of Albizia lebbek, this name has not been used since it was described and its genetic relationship to and within the C. gloeosporioides species complex is unknown.
Colletotrichum gossypii Southw., J. Mycol. 6: 100. 1891.
= Glomerella gossypii Edgerton, Mycologia 1: 119. 1909.
Notes: This species was originally described from the USA and was reported to cause disease symptoms on all parts of cotton plants, but especially the bolls (Southworth 1891, Edgerton 1909). Isolates identified as C. gossypii by Shear & Wood (1907) were reportedly associated with a Glomerella state in culture, and Edgerton (1909) described Glomerella gossypii from diseased, mature cotton plants in the USA Edgerton (1909) discussed differences in ascospore shape between G. gossypii and fruit-rotting isolates of G. cingulata, with G. gossypii having elliptic, not curved ascospores. Von Arx (1957) considered C. gossypii to be a synonym of C. gloeosporioides and von Arx & Müller (1954) regarded G. gossypii to be a synonym of G. cingulata.
Modern authors have recognised two pathogens of cotton, C. gossypii and C. gossypii var. cephalosporioides. Colletotrichum gossypii is reportedly the cause of cotton anthracnose, a damping-off disease of cotton seedlings, and C. gossypii var. cephalosporioides the cause of ramulosis, a disease causing abnormal branching of mature plants (Bailey et al. 1996, Silva-Mann et al. 2005). In a study based on ITS2 sequences, Bailey et al. (1996) found C. gossypii and C. gossypii var. cephalosporioides to be genetically distinct but with both belonging to the C. gloeosporioides species complex. Silva-Mann et al. (2005) also distinguished the two taxa genetically, based on an AFLP analysis. The only DNA sequences available for isolates identified as C. gossypii and C. gossypii var. cephalosporioides are ITS2 and the D2 region of the rDNA LSU, neither of which resolves their relationships within the C. gloeosporioides complex. Whether the seedling pathogen regarded by Silva-Mann et al. (2005) and Bailey et al. (1996) to be C. gossypii represents the species first described from cotton in the USA is not known. The genetic relationship of these apparently biologically specialised fungi requires additional sequences to be generated from authentic isolates with known pathogenicity.
Colletotrichum gossypii var. cephalosporioides A.S. Costa, Bragantia 6: 5. 1946.
≡ Colletotrichum gloeosporioides “var. cephalosporioides” (A.S. Costa) Follin & Mangano, Coton et fibres tropicales 37: 209. 1983. [comb. inval., no full reference to basionym]
Notes: See notes under Colletotrichum gossypii.
* Colletotrichum horii B. Weir & P.R. Johnst., Mycotaxon 111: 21. 2010.
Weir & Johnston (2010) and Xie et al. (2010a) provide descriptions.
Geographic distribution and host range: Associated with fruit and stem disease of Diospyros kaki from China, Japan, and New Zealand. Xie et al. (2010a) noted minor symptoms on inoculated fruit of Capsicum annuum, Musa acuminata, and Cucurbita pepo, but noted that the fungus had never been associated with disease symptoms on these hosts from the field.
Genetic identification: ITS distinguishes C. horii from all other species.
Specimens examined: See Weir & Johnston (2010).
Colletotrichum hymenocallidis Yan L. Yang, Zuo Y. Liu, K.D. Hyde & L. Cai, Fungal Diversity 39: 138. 2009.
Notes: Placed here in synonymy with Colletotrichum siamense. See notes and additional specimens examined under C. siamense. Yang et al. (2009) reported this species as a leaf pathogen of Hymenocallis americana. They distinguished C. hymenocallidis from C. siamense, also described from Hymenocallis, primarily on the basis of a multi-gene phylogeny and differences in colony colour. Although gene selection was appropriate for resolving genetic relationships within the C. gloeosporioides group, Yang et al. (2009) included only five isolates of the C. gloeosporioides complex in their phylogeny. Based on this isolate selection, the C. hymenocallidis isolates were genetically distinct from the C. siamense isolates. However, in our analysis, in which the C. siamense/C. hymenocallidis group is represented by 30 isolates from a wide range of hosts from all over the world, authentic isolates of the two species fall within a monophyletic clade that cannot be further subdivided phylogenetically.
The Latin part of the C. hymenocallidis protologue designates a culture (“Holotypus: Cultura (CSSN2)”) as the holotype but the English citation of the type specimen corrects this apparent mistake, citing CSSN2 as an ex-holotype culture, with the herbarium specimen GZAAS 080001 as the holotype.
Specimen examined: China, Guangxi, Nanning, on Hymenocallis americana leaf spot, coll. Y.L. Yang GZAAS 080001, 19 Jun 2008 (ex-holotype culture of C. hymenocallidis – CBS 125378 = ICMP 18642).
Colletotrichum ignotum E.I Rojas, S.A. Rehner & Samuels, Mycologia 102: 1331. 2010.
Notes: Placed here in synonymy with Colletotrichum fructicola. See notes and additional specimens examined under C. fructicola.
Specimen examined: Panama: Barro Colorado Monument, Tetragastris panamensis leaf endophyte, coll. E.I. Rojas E886, 1 Jun 2004 (ex-holotype culture of C. ignotum – CBS 125397 = ICMP 18646).
Colletotrichum jasmini-sambac Wikee, K.D. Hyde, L. Cai & McKenzie, Fungal Diversity 46: 174. 2011.
Notes: Placed here in synonymy with Colletotrichum siamense based on the ITS, GAPDH, CAL, TUB2, and ACT gene sequences from the ex-holotype culture, deposited in GenBank by Wikee et al. (2011).
Wikee et al. (2011) discussed similarities between C. jasmini-sambac, C. siamense and C. hymenocallidis, three species genetically close in their phylogenetic analysis. The broader range of isolates representing C. siamense in our analysis shows that these species form a single, monophyletic clade that cannot be sensibly subdivided (see notes under C. siamense).
Specimen examined: Vietnam, Cu Chi District, Trung An Ward, on living leaves of Jasminum sambac, Jan. 2009, coll. Hoa Nguyen Thi LLTA–01 (ex-holotype culture of C. jasmini-sambac – CBS 130420 = ICMP 19118).
* Colletotrichum kahawae J.M. Waller & Bridge subsp. kahawae, Mycol. Res. 97: 993. 1993. Fig. 25.
Fig. 25.
Colletotrichum kahawae subsp. kahawae. A, E. ICMP 17905 (ex IMI 361501). B–C. ICMP 17816 (ex IMI 319418 – ex-holotype culture). C. ICMP 17915 (ex CBS 982.69). A–B. Appressoria. C. Conidia. D–E. Cultures on PDA, 10 d growth from single conidia, from above and below. Scale bar C = 20 μm. Scale bar of C applies to A–C.
Waller et al. (1993) provide a description.
Geographic distribution and host range: Known only from Coffea from Africa.
Genetic identification: ACT, CAL, CHS-1, GAPDH, TUB2, SOD2, and ITS sequences are the same as those from C. kahawae subsp. ciggaro. The two subspecies can be distinguished by GS sequences; C. kahawae subsp. kahawae has a 22 bp deletion and a single C to T transition. Collectively, the two subspecies can be distinguished from all other species using ITS sequences alone.
Notes: Colletotrichum kahawae was proposed by Waller et al. (1993) as a name to refer specifically to Colletotrichum isolates causing Coffee Berry Disease (CBD), to taxonomically distinguish these disease-causing isolates from the several other Colletotrichum spp. that can be isolated from coffee plants, including C. coffeanum (see notes under C. coffeanum). Colletotrichum kahawae is an apparently clonal population (Varzea et al. 2002), widespread on coffee in Africa, and with a distinctive growth form and biology (Waller et al. 1993).
In this paper C. kahawae sensu Waller et al. (1993) is reduced to subspecies. Based on ACT, CAL, CHS-1, GAPDH, TUB2, SOD2, and ITS gene sequences the coffee berry pathogen cannot be distinguished from isolates from a wide range of other hosts that are not pathogenic to coffee. Those other isolates are referred to here as C. kahawae subsp. ciggaro. We retain a distinct taxonomic label for the coffee berry pathogen to reflect its biosecurity importance. In addition to its biology, C. kahawae subsp. kahawae can be distinguished metabolically, and genetically using GS gene sequences. Waller et al. (1993) used a metabolic test, an inability to utilise either citrate or tartrate as a sole carbon source, to help characterise isolates as C. kahawae. None of our C. kahawae subsp. kahawae isolates were able to utilise either citrate or tartrate, whereas all of the C. kahawae subsp. ciggaro isolates were able to utilise one or both of these carbon sources (Weir & Johnston 2009). All of the C. kahawae subsp. kahawae isolates share a 22 bp deletion in the glutamine synthetase gene, lacking in the C. kahawae subsp. ciggaro isolates. Note that one of the isolates metabolically and genetically typical C. kahawae subsp. kahawae (CBS 982.69) was reported by Gielink & Vermeulen (1983) to be non-pathogenic to coffee, but we have not independently checked this result.
The isolates we accept as C. kahawae subsp. kahawae show two cultural types, one matching the description of Waller et al. (1993), slow growing, darkly pigmented cultures with conidia developing mostly in the aerial mycelium. The second cultural type grew even more slowly, had little or no pigmentation within the agar, and the colony surface was covered with numerous acervuli and orange conidial masses. Metabolically and genetically both cultural types were the same, and pathogenicity tests showed that the non-pigmented isolates caused CBD (unpubl. data, D. Silva, Centro de Investigação das Ferrugens do Cafeeiro). Rodriguez et al. (1991) reported further variation in cultural appearance amongst CBD causing isolates.
Waller et al. 1993 stated that C. kahawae was not known to form ascospores. However, Gielink & Vermeulen (1983) observed the production of perithecia on coffee berries that had been inoculated with CBD-causing isolates, many months after inoculation and death of the berries. At least one of the isolates that they cited with this biology, CBS 135.30, has the GS sequence typical of C. kahawae subsp. kahawae. Vermeulen et al. (1984) grew cultures from the perithecia that developed on the previously inoculated berries, and found that none were pathogenic to coffee. It is possible that the perithecia developing on inoculated berries reported by Gielink & Vermeulen (1983) were from other Colletotrichum spp. present on the berries before they were inoculated, and represented species distinct from C. kahawae subsp. kahawae. A similar situation has been noted with some of our inoculations, where species present on tissues prior to inoculation, either endophytic or latent, started to sporulate on the dead tissue following inoculation (unpubl. data, B.S. Weir).
Based on ITS sequences, most of the accessions in GenBank identified as C. kahawae and isolated from coffee, match our concept of C. kahawae subsp. kahawae. There are two exceptions, AF534468 (from Malawi) and AY376540 (STE-U 5295 = IMI 319424 = CBS 112985, from Kenya). The Kenyan isolate was cited as C. kahawae in Lubbe et al. (2004). Based on the ITS sequences, and the TUB2 sequence from isolate STE-U 5295 (AY376588), these isolates represent C. siamense.
Specimens examined: Angola, Ganada, on Coffea arabica berry, coll. J.N.M. Pedro 16/65, 2 Jun. 1965 (IMI 310524 = CBS 982.69 = ICMP 17915). Cameroon, on Coffea arabica (IMI 361501 = ICMP 17905). Kenya, Ruiru, Kakuzi Estate, on Coffea arabica young shoots, coll. D.M. Masaba 22/87, 29 Jan. 1987 (ex-holotype culture of C. kahawae – IMI 319418 = ICMP 17816); on Coffea sp., coll. E.C. Edwards, May 1930 (CBS 135.30 = ICMP 17982). Malawi, on Coffea arabica (IMI 301220 = ICMP 17811).
* Colletotrichum kahawae subsp. ciggaro B. Weir & P.R. Johnst., subsp. nov. MycoBank MB563758. Figs 26, 27.
Fig. 26.
Colletotrichum kahawae subsp. ciggaro. A. ICMP 12952. B, D. ICMP 17932 (ex CBS 112984). E, H. ICMP 17931 (ex IMI 359911). C, F. ICMP 18539 – ex-holotype culture. G. ICMP 18531. A–B. Asci and ascospores. C–D. Appressoria. E. Setae. F–H. Conidia. Scale bars A, E = 20 μm. Scale bar of A applies to A–D, F–H.
Fig. 27.
Colletotrichum kahawae subsp. ciggaro. A. ICMP 12953. B. ICMP 18534. C. ICMP 17922 (ex CBS 237.49 – ex-holotype culture of Glomerella cingulata var. migrans). D. ICMP 17932 (ex CBS 112984). E. ICMP 18539 – ex-holotype culture of C. kahawae subsp. ciggaro. F. ICMP 12952 – single ascospore cultures from single conidial isolate. Cultures on PDA, 10 d growth from single conidia, from above and below.
= Glomerella cingulata var. migrans Wollenw., Z. Parasitenk. (Berlin) 14: 262. 1949.
= Glomerella rufomaculans var. vaccinii Shear, Bull. Torrey Bot. Club. 34: 314. 1907.
Etymology: Based on the title of the Jim Jarmusch movie “Coffee and Cigarettes”, referring to the close genetic relationship between C. kahawae subsp. ciggaro and the coffee pathogen C. kahawae subsp. kahawae; ciggaro is Portuguese for cigarette.
Holotype: Australia, on Olea europaea, coll. V. Sergeeva UWS124, 1989, PDD 102232; ex-type culture ICMP 18539.
Colonies grown from single conidia on Difco PDA 75–85 mm diam after 10 d for most isolates, the ex-holotype culture of G. cingulata var. migrans 48–49 mm diam. Aerial mycelium cottony, grey, dense, or in some isolates with dark stromatic masses and associated orange conidial ooze showing through mycelium from agar surface; in reverse agar with pinkish-orange pigments (6B4–7B4), irregular scattered black spots, and variable levels of development of overlying dark grey to green-grey pigments (4C2–5D4), these sometimes in discrete sectors. See notes below about a divergent growth form single ascospore cultures from perithecia in culture. Conidia form on dark-based acervuli, (12–)16–19.5(–29) × (4.5–) 5(–8) μm (av. 17.8 × 5.1 μm, n = 214), cylindric, straight, apex rounded, often tapering slightly towards the base. Appressoria typically cylindric to fusoid in shape, deeply lobed. Perithecia numerous, forming tightly packed clumps, individual perithecia globose, small, about 250 μm diam, with a short ostiolar neck. Asci 55–100 × 10–12 μm, 8–spored. Ascospores (13.5–)17.5–20(–24) × (4–)4.5–5(–6.5) μm (av. 18.8 × 4.8 μm, n = 121), gently curved, tapering to quite narrow, rounded ends, widest point usually towards one end of the spore.
Geographic distribution and host range: Known from Australia, Germany, New Zealand, and South Africa. Both host and geographic range of the isolates we accept in C. kahawae subsp. ciggaro are broad. Genetic identification: ACT, CAL, CHS-1, GAPDH, TUB2, SOD2, and ITS sequences match those from C. kahawae subsp. kahawae. The two subspecies can be distinguished by GS sequences. Collectively, the two subspecies can be distinguished from all other species using ITS sequences alone.
Notes: The authentic isolate of G. cingulata var. migrans (CBS 237.49) differed from all other isolates we accept in C. kahawae subsp. ciggaro by its slower growth rate. Wollenweber & Hochapfel (1949) distinguished Glomerella cingulata var. migrans from G. cingulata var. cingulata on the basis of pathogenicity (G. cingulata var. migrans was pathogenic to Hypericum and not to apple) and because of its slightly longer ascospores and shorter conidia — ascospores average 21 × 4.2 μm versus 18 × 4.6 μm, conidia average 14 × 5.2 μm versus 18 × 5 μm (Wollenweber & Hochapfel 1949). We were unable to produce ascospores from CBS 237.49, the conidia were similar in size to that reported by Wollenweber & Hochapfel (1949), averaging 16.6 × 5.3 μm. However, the average ascospore and conidial lengths of our C. kahawae subsp. ciggaro isolates varied across the range cited by Wollenweber & Hochapfel (1949) for both G. cingulata var. cingulata and G. cingulata var. migrans, the average ascospore length from individual isolates ranging from 16.6 to 20 μm, the average conidial length ranging from 14.9 to 21.2 μm.
Glomerella rufomaculans var. vaccinii was described by Shear (1907) for a fungus isolated from cranberry that was morphologically identical to isolates from apple and other hosts but which appeared to be biologically distinct (Shear 1907, Shear & Wood 1913). A putatively authentic isolate of this species, deposited by Shear in CBS in 1922, matches C. kahawae subsp. ciggaro genetically. Polashock et al. (2009) discussed the diversity of Colletotrichum spp. associated with North American cranberry fruit rots, reporting a close match between their isolates and C. kahawae. Incorporation of their ITS sequences into our alignment confirms this. Whether or not there is a genetically distinct, cranberry specialised taxon within C. kahawae requires additional genes to be sequenced from the cranberry-associated isolates.
Colletotrichum kahawae subsp. ciggaro was referred to as C. gloeosporioides Group B by Johnston & Jones (1997) and Johnston et al. (2005), and as Undescribed Group 1 by Silva et al. (2012b).
Single ascospore isolates derived from perithecia forming in single conidial cultures of the avocado-associated isolates of C. kahawae subsp. ciggaro from New Zealand showed two highly divergent growth forms (Fig. 27F). One typical of the “wild type” (cottony, grey to dark grey aerial mycelium with dark-based acervuli and orange conidial masses visible through the mycelium, in reverse with pinkish-orange pigmentation, in places this masked by irregular patches or sectors of dark grey pigmentation), the other more or less lacking aerial mycelium, the surface of the colony covered with small, pale-based acervuli with bright orange conidial ooze, in reverse bright orange from the conidial ooze. Although common from single ascospores, the bright, conidial cultural type is rarely formed by isolates from nature (unpubl. data). Similar dimorphic cultural types have been observed also from single ascospore isolates from a member of the C. boninense complex, C. constrictum (unpubl. data, P.R. Johnston).
Fig. 28.
Colletotrichum musae. A. ICMP 12930. B. ICMP 18600. C. ICMP 17817 (ex IMI 52264). Cultures on PDA, 10 d growth from single conidia, from above and below.
Other specimens examined: Brazil, on leaves of Miconia sp., coll. R. Barreto RWB1054, 2009 (ICMP 18728). Germany, Berlin-Dahlem, on stem of Hypericum perforatum, Jun. 1937 (ex-holotype culture of Glomerella cingulata var. migrans – CBS 237.49 = ICMP 17922). New Zealand, Auckland, Waitakere Ranges, on leaves of Kunzea ericoides, coll. S. Joshee 5Kun3.10 (ICMP 18741); Auckland, Waitakere Ranges, on leaves of K. ericoides, coll. S. Joshee 7Kun5.2 (ICMP 18534); Auckland, Waitakere Ranges, on leaves of Toronia toru, coll. G. Carroll TOROTO3 (ICMP 18544); Te Puke, on Persea americana fruit rot, coll. W.F.T. Hartill, 19 Jan. 1989 (ICMP 18531); Te Puke, on P. americana fruit rot, coll. W.F.T. Hartill, 8 Feb. 1988 (ICMP 12952); Te Puke, on P. americana fruit rot, coll. W.F.T. Hartill, 28 Sep. 1991 (ICMP 12953). South Africa, Madeira, on Dryandra sp., coll. J.E. Taylor, 1 Apr. 2001 (CBS 112984, as C. crassipes = ICMP 17932). Switzerland, on Dryas octopetala, coll. P. Cannon (IMI 359911 = CBS 12988 = ICMP 17931). USA, on Vaccinium macrocarpum leaves, coll. C.L. Shear, Apr. 1922 (authentic culture of G. rufomaculans var. vaccinii – CBS 124.22 = ICMP 19122).
Colletotrichum manihotis Henn., Hedwigia 43: 94. 1904.
Notes: Anthracnose is an important disease of cassava (e.g. Chevaugeon 1956, Makambila 1994, Fokunang et al. 2000, Owolade et al. 2008), variously referred to Colletotrichum manihotis, Gloeosporium manihotis Henn., Glomerella manihotis (Sacc.) Petr., Glomerella cingulata “f. sp. manihotis”, or C. gloeosporioides “f. sp. manihotis”. The original descriptions of both C. manihotis and Gloeosporium manihotis are of species with short, broad conidia (8–15 × 4–6 μm), and Chevaugeon (1956) regarded all of these cassava-associated fungi as con-specific. However, based on Fokunang et al. (2000), a morphologically highly diverse set of Colletotrichum isolates are associated with diseased plants. There are three GenBank accessions of Colletotrichum from cassava, all from China, and although only ITS sequences are available for these isolates, they appear to represent a single, distinct species within the C. gloeosporioides complex. How these Chinese isolates relate to cassava-associated isolates from other parts of the world is not known.
* Colletotrichum musae (Berk. & M.A. Curtis) Arx, Verh. Kon. Ned. Akad. Wetensch., Afd. Natuurk., Sect. 2 51(3): 107. 1957. Fig. 28.
Basionym: Myxosporium musae Berk. & M.A. Curtis, Grevillea 3: 13. 1874.
Su et al. (2011) provide a description.
Geographic distribution and host range: Found in association with fruit lesions of Musa spp. in many regions.
Genetic identification: ITS sequences separate C. musae from all other species.
Notes: Colletotrichum musae was originally described from North Carolina (Berkeley 1874), and the name was recently epitypified by Su et al. (2011) on the basis of a specimen collected in Florida (ex-epitype culture CBS 116870). Su et al. (2011) cite several strains from Thailand that match their concept of C. musae, and isolates from anthracnose symptoms on banana fruit from several parts of the world are the same based on our study. These isolates form a well-supported clade within the C. gloeosporioides species complex, show low levels of genetic differentiation, and based on ITS sequences are consistent with C. musae sensu Sreenivasaprasad et al. (1996), Nirenberg et al. (2002) and Shenoy et al. (2007). The morphology in culture agrees with the description of Sutton & Waterston (1970).
We have not seen a Glomerella state in culture and none was mentioned by Su et al. (2011). However, Rodriguez & Owen (1992) reported rare production of perithecia from crosses between two of 14 isolates identified as C. musae. It is not known whether the isolates studied by Rodriguez & Owen (1992) match our concept of C. musae genetically, but it is possible that this species behaves in a similar way to some species in the C. acutatum complex, where the sexual morph can be generated in culture under suitable conditions (Guerber & Correll 2001). The name “Glomerella musae”, used by Rodriguez & Owen (1992) and Krauss et al. (2001), has never been validly published.
More than one species of Colletotrichum has been found in association with rotting banana fruit. From isolates with well characterised sequence data these include a species belonging to C. acutatum s. lat. (Sherriff et al. 1994, Johnston & Jones 1997) that is described as C. paxtonii (Damm et al. 2012a, this issue), and C. karstii that belongs to the C. boninense species complex (Damm et al. 2012b, this issue). The latter forms a sexual stage in culture and is known from Musa in South America and Australia, as well as from many other hosts worldwide, often as an endophyte. Species in the C. boninense species complex have been previously confused with C. gloeosporioides s. lat. Greene (1967) referred isolates pathogenic to banana that were not associated with a teleomorph to C. musae, and a second non-pathogenic species that formed fertile ascospores, to C. gloeosporioides. Whether Glomerella musarum Petch, described from leaves of banana and cited as the teleomorph of C. musae by Sutton (1992) and Hyde et al. (2009), is a synonym of C. musae in the sense we use the name here is not known, but seems unlikely given the rare production of perithecia by this species.
Specimens examined: Indonesia, on Musa sp., coll. G. von Becze, Jan. 1931 (CBS 192.31 = ICMP 17923). Kenya, on Musa sapientum, coll. R.M. Nattrass 1850, 1 Jan. 1953 (IMI 52264 = ICMP 17817). New Zealand, Auckland (imported fruit), on Musa sp., coll. P.R. Johnston C1197.1, 24 May 1991 (ICMP 12931; PDD 59100); Auckland (fruit imported from the Phillipines), on Musa sp., coll. S. Bellgard, 5 May 2009 (ICMP 18600); Auckland, Mt Albert Research Centre, Musa sp. spots on green fruit, coll. P.R. Johnston C809.2, 12 Aug. 1987 (ICMP 12930; PDD 46160); Auckland (fruit imported from the Phillipines), on Musa sp., coll. B. Weir, 17 May 2009 (ICMP 18701; PDD 97438). USA, Florida, on Musa sp., coll. M. Arzanlou A-1 (ex-epitype culture of C. musae – CBS 116870 = ICMP 19119).
Glomerella musarum Petch, Ann. Roy. Bot. Gard. Peradeniya 6(3): 223. 1917.
Notes: See notes under C. musae.
* Colletotrichum nupharicola D.A. Johnson, Carris & J.D. Rogers, Mycol. Res. 101: 647. 1997. Fig. 29.
Fig. 29.
Colletotrichum nupharicola. A. ICMP 17939 (ex CBS 470.96 – ex-holotype culture). B. ICMP 17938 (ex CBS 469.96). C. ICMP 18187 (ex CBS 472.96). Cultures on PDA, 10 d growth from single conidia, from above and below.
Johnson et al. (1997) provide a description.
Geographic distribution and host range: Known only from the USA, on the aquatic plants Nuphar and Nymphaea spp.
Genetic identification: One of the two ITS haplotypes of C. nupharicola is identical with C. queenslandicum. All other genes distinguish this species well from other species in the C. gloeosporioides complex.
Notes: Sequence data from the ex-holotype culture of C. nupharicola places it within the C. gloeosporioides complex, genetically close to C. fructicola and C. alienum in the Musae clade. This apparently host-specific species and has a distinctive, slow growth in culture and massive conidia (Johnson et al. 1997).
Johnson et al. (1997) compare C. nupharicola with another water plant pathogen, C. nymphaeae, that is epitypified and shown to belong to the C. acutatum species complex by Damm et al. (2012a, this issue).
Specimens examined: USA, Washington, King Co., on Nuphar lutea subsp. polysepala, coll. D.A. Johnson A-7, Oct. 1993 (CBS 469.96 = ICMP 17938); Washington, Yakima Co., on N. lutea subsp. polysepala, coll. D.A. Johnson A-2, Oct. 1993 (ex-holotype culture – CBS 470.96 = ICMP 17939); Rhode Island, on Nymphaea ordorata, coll. R.D. Goos RDG-291, 1979 (CBS 472.96 = ICMP 18187).
Gloeosporium pedemontanum Pupillo, Ann. Sperim. Agrar. n.s. 6: 57. 1952.
Notes: Placed here in synonymy with C. gloeosporioides. See notes under C. gloeosporioides.
Specimen examined: Italy, on Citrus limon juice, coll. G. Goidánich, 1951 (ex-holotype culture of G. pedemontanum – CBS 273.51 = ICMP 19121).
* Colletotrichum psidii Curzi, Atti dell’Istituto Botanico dell’Università di Pavia, ser. 3, 3: 207. 1927. Fig. 30.
Fig. 30.
Colletotrichum psidii (ICMP 19120, ex CBS 145.29 – authentic culture). Cultures on PDA, 10 d growth from single hyphal tips, from above and below.
Colonies grown from single conidia on Difco PDA 58–63 mm diam after 10 d, aerial mycelium dense, cottony to felted, uniform in height, white to off-white; in reverse uniformly pale creamy yellow (2A2–2A3) or in some cultures becoming dull greyish yellow (2D2–2E2) towards the centre. No conidiogenous cells or conidia seen.
Geographic distribution and host range: Known from a single isolate, from Psidium from Italy.
Genetic identification: Although known from only one isolate, ITS sequences separate C. psidii from all other taxa.
Notes: A putatively authentic isolate of this species, deposited in CBS by Curzi shortly after publication of C. psidii, represents a genetically distinct species within the Kahawae clade. The only available culture is stale, no longer forming conidia. Curzi (1927) describes the conidia as 12–15 × 3.5–4.5 μm, cylindric with rounded ends, straight or rarely slightly curved.
Anthracnose diseases have been noted for Psidium spp. (guava) from several tropical regions of the world (e.g. MacCaughey 1917, Venkatakrishniah 1952, Liu 1972, Misra 2004). It is likely that several Colletotrichum spp. are associated with guava fruit rots. Whether the fungus described by Curzi from an Italian botanical garden represents one of the species causing a guava disease in the tropics is not known. All other members of the Kahawae clade are predominantly tropical, so perhaps this fungus was introduced to Italy along with its host plant. Misra (2004) uses C. psidii to refer to a Colletotrichum species with curved conidia.
One other species has been described from this host, Glomerella psidii (basionym Gloeosporium psidii), the relationship of this species to C. psidii remains unknown. A new species on Psidium guajava, C. guajavae, belonging to the C. acutatum species complex, is described elsewhere in this volume (Damm et al. 2012a).
Specimen examined: Italy, Rome, on Psidium sp., coll. M. Curzi (authentic culture of C. psidii – CBS 145.29 = ICMP 19120).
Glomerella psidii (Delacr.) J. Sheld., Bull. West Virginia Agric. Exp. Sta. 104: 311. 1906.
Basionym: Gloeosporium psidii Delacr., Bull. Soc. Mycol. France. 19: 144. 1903.
Notes: Sheldon (1906) produced perithecia in culture from isolates he considered typical of Gloeosporium psidii and on this basis recombined the species described by Delacroix (1903) in Glomerella. The relationship of G. psidii to Colletotrichum psidii, also described from guava, is not known. See notes under C. psidii.
* Colletotrichum queenslandicum B. Weir & P.R. Johnst., nom. nov. et stat. nov. MycoBank MB563593. Fig. 31.
Fig. 31.
Colletotrichum queenslandicum. A, C, E. ICMP 1778 – ex-epitype culture. B, F. ICMP 1780. D, G. ICMP 12564. H. ICMP 18705. A–B. Appressoria. C–D. Conidia. E–H. Cultures on PDA, 10 d growth from single conidia, from above and below. Scale bar A = 20 μm. Scale bar of A applies to A–D.
Basionym: Colletotrichum gloeosporioides var. minus Simmonds, Queensland J. Agric. Anim. Sci. 25: 178A. 1968. [as var. minor]
Etymology: based on the region from which the type specimen of this species was collected.
Holotype: Australia, Queensland, Ormiston, on Carica papaya, coll. J.H. Simmonds, Oct. 1965, IMI 117612.
Epitype: Australia, Queensland, Brisbane, on Carica papaya, coll. J.H. Simmonds 11663C, Sep. 1965, epitype here designated PDD 28797; ex-epitype culture ICMP 1778.
Colonies grown from single conidia on Difco PDA 62–74 mm diam after 10 d, aerial mycelium either dense, cottony, uniform, grey, or with aerial mycelium lacking, towards centre of colony with numerous, small acervuli with dark bases and orange conidial ooze; in reverse cultures with copious aerial mycelium uniformly dark grey (1F2), those with little aerial mycelium having a pinkish brown (8B4) pigment within the agar, the dark bases of the acervuli and the colour of the conidial ooze visible through the agar. Conidia (12–)14.5–16.5(–21.5) × (3.5–)4.5–5(–6) μm (av. 15.5 × 4.8 μm, n = 96), cylindric, straight, sometimes slightly constricted near centre, ends broadly rounded. Appressoria about 6–12 μm diam., globose to short-cylindric, rarely lobed. Perithecia not seen.
Geographic distribution and host range: Known from Carica papaya and Persea americana from Queensland, Australia, and from Coffea berries from Fiji. Simmonds (1965) reported from Australia what he considered to be the same fungus also from Mangifera indica, Malus sylvestris, and “many other hosts”.
Genetic identification: ITS sequences do not separate C. queenslandicum from some C. fructicola, some C. siamense, and some C. tropicale isolates. It is best distinguished from these taxa using TUB2, GAPDH, or GS.
Notes: The ex-type cultures cited by Simmonds (1968) are no longer in storage at BRIP in Queensland (R. Shivas, pers. comm.) and presumably lost. However, we do have two cultures identified as C. gloeosporioides var. minus by Simmonds and isolated from the same host from the same locality as the holotype (Simmonds isolates 16633C and 1647A2), that had been sent to Joan Dingley in 1965 and subsequently stored in the ICMP culture collection. The culture selected here as epitype (Simmonds 11663C = ICMP 1778) matches the Simmonds (1965) description of this fungus as having “an abundance of aerial mycelium in culture”. Our conidial measurements from ICMP 1778 and 1780 are broader than those given by Simmonds (1965), but he does note that “Confusion can occur between narrower strains of C. gloeosporioides and broader strains of C. gloeosporioides var. minus...”. Simmonds (1965) also notes that perithecia may rarely be seen in cultures of some isolates.
The isolates accepted here as C. queenslandicum are genetically distinct within the Musae clade of C. gloeosporioides s. lat. Colletotrichum minus Zimm. (1901) requires that we propose a nom. nov. for this fungus at species rank.
Simmonds (1965) considered C. gloeosporioides var. minus to be the conidial state of Glomerella cingulata var. minor Wollenw. Wollenweber & Hochapfel (1949) used the name Gloeosporium elasticae Cooke & Massee for the conidial state of G. cingulata var. minor, the type specimens for both names being from Ficus. Simmonds (1965) noted that it was not possible to transfer G. elasticae to Colletotrichum because Colletotrichum elasticae had already been published for a different fungus. However, rather than proposing a nom. nov. for Gloeosporium elasticae, he described C. gloeosporioides var. minus as a new variety, with a different type specimen. Glomerella cingulata var. minor is genetically distinct from the specimen Simmonds chose as the type of C. gloeosporioides var. minus, see notes under G. cingulata var. minor.
Other specimens examined: Australia, Queensland, Brisbane, on Carica sp., coll. J.H. Simmonds 16347A2 (ICMP 1780, dried culture stored as PDD 28797); Queensland, Home Hill, on Persea americana, coll. L. Coates 22516, Feb. 1983 (ICMP 12564). Fiji, on Coffea sp. berry, coll. R. Gounder, Apr. 1988 (ICMP 18705).
Glomerella rufomaculans var. vaccinii Shear, Bull. Torrey Bot. Club. 34: 314. 1907.
Notes: Placed here in synonymy with Colletotrichum kahawae subsp. ciggaro. See notes under C. kahawae subsp. ciggaro. Note that Saccardo & Trotter (1913) place Shear’s variety in Glomerella fructigena (Clint.) Sacc., a rarely used species name, placed in synonymy with G. cingulata by von Arx & Müller (1954).
Specimen examined: USA, on Vaccinium macrocarpum leaves, coll. C.L. Shear, Apr. 1922 (authentic isolate of G. rufomaculans var. vaccinii – CBS 124.22 = ICMP 19122).
*Colletotrichum salsolae B. Weir & P.R. Johnst., sp. nov. MycoBank MB563589. Fig. 32.
Fig. 32.
Colletotrichum salsolae. A, C–H. ICMP 19051 – ex-holotype culture. B. BPI 878740 – holotype. A. Cultures on PDA, 10 d growth from single conidia, from above and below. B. Lesion on stem, dried type specimen. C. Conidiogenous cells. D–E. Conidia. F–G. Appressoria. Scale bars B = 1 mm, C = 20 μm. Scale bar of C applies to C–G.
= Colletotrichum gloeosporioides “f. sp. salsolae” (Berner et al. 2009).
Etymology: Based on C. gloeosporioides “f. sp. salsolae”, referring to the host from which this fungus was originally collected.
Holotype: Hungary, on Salsola tragus, coll. D. Berner [specimen from plants inoculated with strain 96-067, originally collected I. Schwarczinger & L. Vajna on Salsola tragus from Bugac, near Kiskunsag National Park, 1996], BPI 878740; ex-holotype culture ICMP 19051.
Colonies grown from single conidia on Difco PDA 38–42 mm diam after 10 d, aerial mycelium sparse, cottony, pale grey, surface of colony dark, a more or less continuous layer of acervulus-like structure with deep orange brown conidial masses and numerous setae; in reverse dark purplish-black near centre of colony, dark olivaceous near the margin. Conidia (10–)14–16.5(–20.5) × (4.5–) 5.5–6(–7.5) μm (av. 15.3 × 5.8 μm, n = 24), highly variable in size and shape, subglobose to long-cylindric, apex usually broadly rounded, small truncate scar at base. Conidiogenous cells 13–18 × 4–6.5 μm, cylindric to flask-shaped, tapering at apex to narrow, phialidic conidiogenous locus, wall at base often encrusted with dark brown material. Appressoria sparsely developed, cylindric to elliptic, simple; many putatively partially developed appressoria, similar in shape to those with dark and thick walls and also with an appressorial pore, but the wall remains thin and only slightly pigmented. Perithecia not seen.
Geographic distribution and host range: Known from throughout the geographic range of Salsola tragus (Berner et al. 2009), reported in nature only from Salsola spp.
Genetic identification: ITS sequences of C. salsolae are very close to C. alienum and some C. siamense isolates. These species can be distinguished using TUB2 or GAPDH.
Notes: Isolates of C. gloeosporioides pathogenic to Salsola tragus were reported by Schwarczinger et al. (1998) and referred to as C. gloeosporioides “f. sp. salsolae” by Berner et al. (2009). Although mildly pathogenic to a wide range of hosts in glasshouse pathogenicity tests, this fungus causes severe disease only on Salsola spp. with the exception of S. orientalis, S. soda, and S. vermiculata (Berner et al. 2009).
Colletotrichum salsolae belongs to the Musae clade, and although genetically close to several other species, it is biologically and morphologically distinctive.
Other specimen examined: Hungary, additional isolate of strain selected as the holotype, recovered from inoculated Glycine max plants (MCA 2498 = CBS 119296 = ICMP 18693).
* Colletotrichum siamense Prihastuti, L. Cai & K.D. Hyde, Fungal Diversity 39: 98. 2009. Fig. 33.
Fig. 33.
Colletotrichum siamense. A. ICMP 18642 (ex CBS 125378 – ex-holotype culture of C. hymenocallidis). B. ICMP 18578 (ex MFLU 090230 – ex-holotype culture of C. siamense). C. ICMP 12565. D. ICMP 18574 (ex DAR 76934). E. ICMP 18618 (ex HKUCC 10881). F. ICMP 18121. Cultures on PDA, 10 d growth from single conidia, from above and below.
= Colletotrichum jasmini-sambac Wikee, K.D. Hyde, L. Cai & McKenzie, Fungal Diversity 46: 174. 2011.
= Colletotrichum hymenocallidis Yan L. Yang, Zuo Y. Liu, K.D. Hyde & L. Cai, Fungal Diversity 39: 138. 2009.
Descriptions of this species are provided by Prihastuti et al. (2009), Wikee et al. (2011), and Yang et al. (2009).
Geographic distribution and host range: Colletotrichum siamense was originally described from coffee from Thailand, but our concept of this species is biologically and geographically diverse, found on many hosts across several tropical and subtropical regions.
Genetic identification: ITS sequences do not reliably separate C. siamense from C. alienum, C. fructicola, or C. tropicale. These species are best distinguished using CAL or TUB2.
Notes: Yang et al. (2009) and Wikee et al. (2011) discussed genetic and morphological differences between C. siamense, C. jasmini-sambac, and C. hymenocallidis. However, both studies used a limited set of isolates within the C. gloeosporioides complex, making interpretation of the genetic differences difficult. The morphological differences they described are commonly seen as within-species variation in other Colletotrichum spp. In our analysis, C. siamense is represented by 30 isolates from a wide range of hosts from several tropical regions, and forms a monophyletic clade that cannot be further subdivided genetically. Variation in cultural appearance is broad but in part this probably reflects the different conditions under which the isolates had been stored. Shape and size of appressoria, and the characteristically small conidia are similar in all isolates.
Based on matching translation elongation factor (TEF) and TUB2 sequences, isolates referred by Rojas et al. (2010) to Colletotrichum sp. indet. 2 also represent C. siamense. Note that TEF data was excluded from our phylogenetic analyses because the TEF gene tree was often incongruent with the trees from the other genes that we sequenced. For example, compare our isolate ICMP 17797 (GenBank GU174571) with isolates Rojas et al. (2010) cite as Colletotrichum sp. indet. 2, V1H1_1 (GenBank GU994297) and 7767 (GenBank GU994298).
The C. siamense protologue designates the holotype as MFLU 090230, but the culture derived from holotype as “BCC” with no specimen number. The ex-holotype culture is listed as BDP-I2 in Table 1 of Prihastuti et al. (2009) but not in the description of the species. Strain BDP-I2 was obtained from the authors (Prihastuti et al. 2009) for this study and deposited as ICMP 18578.
Specimens examined: Australia, New South Wales, Murwillumbah, on Persea americana fruit rot, coll. L. Coates 23695, 1 Apr. 1990 (ICMP 12567); New South Wales, Muswellbrook, on Pistacia vera (DAR 76934 = ICMP 18574); Queensland, Mt Tamborine, on Persea americana fruit rot, coll. L. Coates T10-1, 1 Sep. 1993 (ICMP 12565). China, Guangxi, Nanning, on Hymenocallis americana leaf spot, coll. Y.L. Yang CSSN2, 19 Jun. 2008 (ex-holotype culture of C. hymenocallidis – CBS 125378 = ICMP 18642); Guangxi Province, Nanning, on H. americana leaf, coll. Y.L. Yang CSSN3 (CBS 125379 = ICMP 18643). Nigeria, Ibadan, on Dioscorea rotundata seed, coll. M. Abang CgS2 (ICMP 18121); Ibadan, on D. rotundata seed, coll. M. Abang CgS6 (ICMP 18117); Ibadan, on Commelina sp. leaf, coll. M. Abang Cg29 (ICMP 18118). South Africa, on Carica papaya fruit, coll. L. Korsten PMS 1 (ICMP 18739). on Persea americana, coll. L. Korsten Cg227 (ICMP 18570); on Persea americana, coll. L. Korsten Cg231 (ICMP 18569). Thailand, Chiang Mai, Mae Lod Village, on Coffea arabica berries, coll. H. Prihastuti BPD-I2, 12 Dec. 2007 (ex-holotype culture of C. siamense – MFLU 090230 = ICMP 18578). Kanchanaburi, on Capsicum annuum, P.P. Than Ku4 (HKUCC 10884 = ICMP 18575); Nakhonpathon, on C. annuum, coll. P.P. Than Ku8 (HKUCC 10881 = ICMP 18618). USA, Florida, on Vitis vinifera leaf, coll. N. Peres ssgrape 10 (ICMP 18572); Florida, on Fragaria × ananassa crown, coll. N. Peres strawberry 6 (ICMP 18571); Florida, on V. vinifera leaf, coll. N. Peres DI-grape-6 (ICMP 18573); North Carolina, Wilkes County, on Malus domestica fruit, coll. T. Sutton LD Cg12 2001 (ICMP 17795); North Carolina, Johnston County, on M. domestica fruit, coll. T. Sutton GD 8 2002 (ICMP 17791); North Carolina, Johnston County, on M. domestica fruit, coll. T. Sutton GD 7 2002 (ICMP 17797); Alabama, on M. domestica fruit, coll. T. Sutton AL 1 2001 (ICMP 17785). Vietnam, Cu Chi District, Trung An Ward, on living leaves of Jasminium sambac, Jan. 2009, coll. Hoa Nguyen Thi LLTA–01 (ex-holotype culture of C. jasmini-sambac – CBS 130420 = ICMP 19118).
* Colletotrichum theobromicola Delacr., Bull. Soc. Mycol. France. 31: 191. 1905. Fig. 34.
Fig. 34.
Colletotrichum theobromicola. A. ICMP 17957 (ex MUCL 42294 – ex-holotype culture of C. gloeosporioides f. stylosanthis). B. ICMP 17927 (ex CBS 142.31 – ex-epitype culture of C. fragariae). C. ICMP 17958 (ex MUCL 42295). D. ICMP 17895. E. ICMP 18567. F. ICMP 18566. Cultures on PDA, 10 d growth from single conidia, from above and below.
= Colletotrichum fragariae A.N. Brooks, Phytopathology 21: 113. 1931.
= Colletotrichum gloeosporioides f. stylosanthis Munaut, Mycol. Res. 106: 591. 2002.
= Colletotrichum gloeosporioides f. stylosanthis “f. sp. stylosanthis” (Munaut et al. 2002).
= Colletotrichum gloeosporioides f. stylosanthis “f. sp. guianensis” (Munaut et al. 2002).
A modern description of this species is provided by Rojas et al. (2010).
Geographic distribution and host range: Broadly distributed in tropical and subtropical regions on a wide range of hosts.
Genetic identification: ITS sequences distinguish C. theobromicola from all other species.
Notes: The ex-epitype culture of Colletotrichum fragariae, the ex-neotype culture of C. theobromicola, and the ex-holotype culture of C. gloeosporioides f. stylosanthis, selected by Buddie et al. (1999), Rojas et al. (2010), and Munaut et al. (2002) respectively, belong in a clade that we accept genetically as a single species. Also in this clade are authentic isolates of C. gloeosporioides f. stylosanthis “f. sp. stylosanthis” and C. gloeosporioides f. stylosanthis “f. sp. guianensis” (but see notes under C. gloeosporioides f. stylosanthis).
Colletotrichum theobromicola as accepted here contains several putatively specialised pathogens, including the pathogen of strawberry runners described by Brooks (1931) as C. fragariae, and the pathogens of Stylosanthes referred to as C. gloeosporioides f. stylosanthis (Munaut et al. 2002). Future studies may show that the species should be segregated based on their pathogenicity to specific hosts. See also notes under C. fragariae and C. gloeosporioides f. stylosanthis.
Munaut et al. (2002) distinguished C. gloeosporioides f. stylosanthis from isolates they considered to represent C. gloeosporioides f. gloeosporioides because of 2 additional C’s at positions 93 and 94 in the ITS1 region, giving a string of 7 C’s at this position. This characteristic feature of the ITS-1 is found also in the ex-neotype isolate of C. theobromicola, the ex-epitype isolate of C. fragariae and all other isolates of C. theobromicola, although a few isolates have 3 additional C’s rather than 2. None of the other isolates that we sampled from the C. gloeosporioides species complex have this characteristic string of C’s.
Rojas et al. (2010) provide a description for their concept of C. theobromicola, MacKenzie et al. (2008) for C. fragariae, and Irwin & Cameron (1978) for C. gloeosporioides f. stylosanthis “f. sp. stylosanthis” (as C. gloeosporioides Type A) and C. gloeosporioides f. stylosanthis “f. sp. guianensis” (as C. gloeosporioides Type B). In cultural appearance the isolates we accept in this species are variable, from the very dark ex-neotype isolate of C. theobromicola to the slow-growing, pale coloured C. gloeosporioides f. stylosanthis “f. sp. guianensis”. None of the isolates that we examined formed perithecia in culture. All had conidia tapering slightly towards each end, this more pronounced towards the base, matching the description of C. fragariae by Gunnell & Gubler (1992), who regarded the conidial shape as distinctive for the species. Some of the isolates studied by Gunnell & Gubler (1992) were included in the study of MacKenzie et al. (2008), their genetic concept of C. fragariae matching ours.
See also notes under C. fragariae and C. gloeosporioides f. stylosanthis.
Specimens examined: Australia, Queensland, Townsville, on Stylosanthes viscosa, coll. J.A.G. Irwin 21365 (HM335), 1976 (ex-holotype culture of C. gloeosporioides f. stylosanthis – MUCL 42294 = ICMP 17957); Samford, on Stylosanthes guianensis, coll. J.A.G. Irwin 21398 (HM336), 1979 (MUCL 42295 = ICMP 17958); New South Wales, Olea europaea fruit, coll. V. Sergeeva UWS 128, 21 Apr. 2008 (ICMP 18566); New South Wales, O. europaea fruit, coll. V. Sergeeva UWS 130, 21 Apr. 2008 (ICMP 18565); New South Wales, O. europaea fruit, coll. V. Sergeeva UWS 98, 8 Apr. 2008 (ICMP 18567). Israel, on Limonium sp. leaf lesion, coll. S. Freeman P1 (cited in Maymon et al. 2006) (ICMP 18576). Mexico, on Annona diversifolia, coll. R. Villanueva-Aroe Gro-7, Jul. 2003 (ICMP 17895). New Zealand, Kerikeri, on Acca sellowiana, coll. M.A. Manning MM317, 1 Feb. 2004 (ICMP 15445). Panama, Chiriqui Province, San Vicente, on Theobroma cacao pod lesion, coll. E.J. Rojas ER08-9, Jan. 2008 (CBS 125393 = ICMP 18650); Chiriqui Province, Escobal, on T. cacao leaf spot, coll. E.J. Rojas GJS 08-50, Jan. 2008 (ex-neotype culture of C. theobromicola – CBS 124945 = ICMP 18649). USA, Florida, Dover, Plant City, on Fragaria × ananassa, coll. S. MacKenzie 326-1, 1988 (ICMP 17099); Florida, Lake Alfred, on Quercus sp. leaf, coll. S. MacKenzie LA-oak-13, 2002 (ICMP 17100); Louisiana, on F. vesca, 1985 (IMI 348152 = ICMP 17814); Florida, on F. × ananassa, coll. A.N. Brooks, 1931 (ex-epitype culture of C. fragariae – CBS 142.31 = ICMP 17927).
* Colletotrichum ti B. Weir & P.R. Johnst., sp. nov. MycoBank MB563594. Figs 35, 36.
Fig. 35.
Colletotrichum ti. A. PDD 24881 – holotype. B. PDD 30206. C, D, F, H. ICMP 4832 – ex-holotype culture. E, G. ICMP 19444. A–B. Lesions on dried herbarium specimens. C–E. Appressoria. F. Conidia. G. Conidiogenous cells. H. Ascospores. Scale bars A = 1 mm, C = 20 μm. Scale bar of A applies to A–B, scale bar of C applies to C–H.
Fig. 36.
Colletotrichum ti. A. ICMP 19444. B. ICMP 4832 – ex-holotype culture. C. ICMP 5285. Cultures on PDA, 10 d growth from single conidia, from above and below.
Etymology: Based on the Maori name for Cordyline australis, tī.
Holotype: New Zealand, Taupo, on Cordyline sp., coll. J.M. Dingley 65187, Sep. 1965, PDD 24881; ex-holotype culture ICMP 4832.
Leaf spots oblong to elliptic in shape, up to about 1 × 2 mm, sometimes coalescing when close together on a leaf, pale grey and necrotic in the centre with a reddish margin; acervuli numerous, base pale to dark grey, with scattered, dark brown setae about 50–80 μm long. Perithecia not seen on infected leaves. Freshly isolated colonies on Difco PDA 50–55 mm diam after 10 d, margin slightly irregular and feathery, aerial mycelium lacking from ex-holotype culture, when present fine, cottony, pale grey, surface of colony dark towards the centre, pale pinkish orange (7A6) towards margin, conidia forming over all parts of culture, mostly not associated with well differentiated acervuli, setae not observed; in reverse purple (12E3) near centre, orange outside, sometimes with concentric rings of grey pigment. Conidiogenous cells cylindric, mostly 15–25 × 3.5–4.5 μm, towards centre of colony arranged in closely packed palisade, towards margin the conidiophores with a much looser structure, irregularly branched, conidiogenous loci at apex and often also at septa. Conidia (11.5–)14–17.5(–23.5) × (4–)5–5.5(–7.5) μm (av. 16 × 5.2 μm, n = 53), cylindric, ends broadly rounded, sometimes tapering towards basal end. Appressoria often narrow-cylindric, often tapering towards apex, sometimes irregularly lobed. Perithecia developing in small numbers in culture after about 4 wk, solitary, scattered across plate, dark-walled, globose with well-developed, tapering ostiolar neck. Asci (60–)65–75(–78) × (10–) 11(–12) μm (av. 69.6 × 11 μm, n = 5), cylindric to subfusoid, 8–spored. Ascospores (14.5–)15.5–16.5(–19) × (4.5–)5–5.5(–6) μm (av. 15.9 × 5.2 μm, n=18), broad-cylindric, ends broadly rounded, not tapering to the ends, in side view mostly flat on one side, often slightly curved.
Geographic distribution and host range: Known only from Cordyline spp. from New Zealand.
Genetic identification: ITS sequences do not distinguish C. ti from C. aotearoa. The two species can be distinguished using TUB2 or GAPDH.
Notes: A member of the Kahawae clade, this fungus causes a leaf spot of Cordyline spp. in New Zealand. It is genetically distinct from C. cordylinicola, described from Cordyline fruticosa from Thailand. Based on the published description of C. cordylinicola (Phoulivong et al. 2011) the two fungi are morphologically similar. Inoculation tests using culture ICMP 5285 when freshly isolated (J.M. Dingley, unpublished data), showed it to be pathogenic to Cordyline australis, forming spots on leaves 2 wk after inoculation, but causing no symptoms on apple, even after wounding.
Although only four of the specimens examined have been compared genetically, all of the cited specimens examined match in terms of associated symptoms and conidial size and shape. A specimen from Cordyline banksii (PDD 78360) has narrower conidia, forms perithecia on the infected leaves, and perhaps represents a different species. Specimens accepted here as C. ti were referred to Glomerella cingulata by Laundon (1972).
The appearance in culture varies between isolates. The J.M. Dingley cultures, first isolated in the mid-1960’s, have dense, felted aerial mycelium and limited conidial production; one has a much slower growth rate than the more recent collections.
Other specimens examined: New Zealand, Auckland, on Cordyline australis, coll. J.M. Dingley 6653, Mar. 1966 (PDD 30206; ICMP 5285); Taranaki, New Plymouth, Duncan and Davies Nursery, on C. australis × C. banksii leaf spots, coll. G.F. Laundon LEV 3343, 26 May 1969 (PDD 50634); Taranaki, New Plymouth, Duncan and Davies Nursery, on C australis × C. banksii leaf spots, coll. G.F. Laundon, 26 May 1969 (PDD 26775); Waikato, Cambridge, Anton Nursery, on C. australis leaf spots, coll. L.A. Houghton, 23 Jul. 1992 (PDD 61219; ICMP 19444).
* Colletotrichum tropicale E.I. Rojas, S.A. Rehner & Samuels, Mycologia 102: 1331. 2010. Fig. 37.
Fig. 37.
Colletotrichum tropicale. A. ICMP 18653 (ex CBS 124949 – ex-holotype culture). B. ICMP 18651 (ex CBS 124943). C. ICMP 18672 (ex MAFF 239933). Cultures on PDA, 10 d growth from single conidia, from above and below.
Rojas et al. (2010) provide a description.
Geographic distribution and host range: Rojas et al. (2010) noted that C. tropicale has been isolated from a wide range of hosts in forests in tropical America, from rotting fruit as well as leaf endophytes. We include also an isolate from tropical Japan, from Litchi chinensis leaves.
Genetic identification: ITS sequences do not separate C. tropicale from some C. siamense or some C. queenslandicum isolates. Colletotrichum tropicale is best distinguished using TUB2, CHS-1, GS, or SOD2.
Notes: Colletotrichum tropicale is genetically close to C. siamense and the two species share a number of morphological features; slow growth in culture, short and broad conidia with broadly rounded ends and often slightly constricted near the centre, and simple appressoria.
Specimens examined: Japan, Okinawa, on Litchi chinensis leaf (MAFF 239933 = ICMP 18672). Panama, Barro Colardo Monument, on Theobroma cacao leaf, coll. E.I. Rojas, L.C. Mejía, Z. Maynard 5101, 2008 (ex-holotype culture – CBS 124949 = ICMP 18653); Escobal, Chiriqui, on Annona muricata fruit rot, coll. E.I. Rojas GJS 08-42 (CBS 124943 = ICMP 18651).
* Colletotrichum xanthorrhoeae R.G. Shivas, Bathgate & Podger, Mycol. Res. 102: 280. 1998. Fig. 38.
Fig. 38.
Colletotrichum xanthorrhoeae. ICMP 17903 (ex BRIP 45094 – ex-holotype culture). A. Cultures on PDA, 10 d growth from single conidia, from above and below. B. Culture on PDA at 4 wk showing sectoring with variation in pigmentation and growth form. C–D. Asci and ascospores. E. Perithecial wall in squash mount. Scale bar C = 20 μm. Scale bar of C applies to C–E.
Shivas et al. (1998) provide a description. One of the isolates we examined (ICMP 17820) formed fertile perithecia in culture, a feature not mentioned in the original description. Perithecia are dark-walled, globose with a prominent, narrow neck, wall comprising several layers of pseudoparenchymatous cells 8–15 μm diam, with several layers of densely packed hyphae outside this. Asci 75–100 × 10–12 μm, 8-spored. Ascospores (17–)18.5–20(–22) × (5–)5.5–6 μm (av. 19.4 × 5.6 μm, n = 24), more or less elliptic, tapering to narrow, rounded ends, in side view flattened on one side, but generally not curved.
Genetic identification: ITS sequences distinguish C. xanthorrhoeae from all other species.
Notes: This pathogen of Xanthorrhoea has a distinctive morphology, with a very slow growth rate in culture and large conidia which taper towards the basal end. The ascospore shape is distinct to that of most taxa within the C. gloeosporioides group, which typically have bent or curved ascospores.
Specimens examined: Australia, Western Australia, Melville, on Xanthorrhoea preissii leaf spots, coll. F.D. Podger, Jan. 1994 (ex-holotype culture – BRIP 45094 = ICMP 17903 = CBS 127831); Queensland, Cunningham’s Gap, Main Ranges National Park, on Xanthorrhoea sp. leaf spot (IMI 350817a = ICMP 17820).
DISCUSSION
The species that we accept in the Colletotrichum gloeosporioides species complex together form a strongly supported clade in the Colletotrichum ITS gene tree (fig. 1 in Cannon et al. 2012, this issue). All species are micro-morphologically typical of C. gloeosporioides sensu von Arx (1970) and Sutton (1992). However, morphology alone cannot unequivocally place an isolate in this complex, making the ITS particularly important for identification at the species complex level in Colletotrichum. For example, members of the C. boninense species complex (Damm et al. 2012b, this issue) and C. cliviae (Yang et al. 2009) are micro-morphologically similar to species in the C. gloeosporioides complex but genetically distinct (Cannon et al. 2012, this issue). The utility of ITS sequences is enhanced by their strong representation in GenBank, but this can also be a problem. Nilsson et al. (2006) summarised the frequency of inaccurately annotated data in GenBank. The diversity of taxonomic concepts around the name C. gloeosporioides makes this a particular problem. This is illustrated by the phylogeny presented by Hyde et al. (2010), based on GenBank accessions of ITS sequences identified as C. gloeosporioides and Glomerella cingulata, that shows the taxa represented belong to many species in different Colletotrichum species complexes. See notes under C. boehmeriae, C. crassipes, and C. kahawae subsp. kahawae for specific examples of misidentified GenBank accessions.
The species we accept are based on a phylogenetic species concept, all species forming strongly supported, monophyletic clades within our multigene phylogenies. However, not all terminal clades are recognised as named species. In most cases any well supported, within-species phylogenetic structure evident in the multi-gene phylogeny is not resolved consistently in all gene trees. This lack of congruence between gene trees is a signal that the diversity being sampled is below the species level, according to the logic of the genealogical concordance phylogenetic species recognition (GCPSR) concept (Taylor et al. 2000). Although the concatenation of gene sequences is a convenient way to present multigene data, it masks discordance between individual gene phylogenies. An alternative method, using a species-tree approach (Figs 3, 4B, 5B) combines multi-gene data from multiple isolates hypothesised to represent a single species, so that the evolutionary history of the species rather than that of individual isolates is estimated. Fig. 3, shows the results of such an analysis for the C. gloeosporioides complex, Figs 4B and 5B show relationships within the Musae and Kahawae clades respectively, at an expanded scale. Posterior probabilities for some of the speciation events are low, particularly within the Musae and Kahawae clades. This may be because although the species-trees algorithms account for incomplete lineage sorting (Heled & Drummond 2010, Chung & Ané 2011), most do not compensate for horizontal gene transfer, reassortment, or introgression. Hybridisation could also result in discordant gene phylogenies. Hybrids are known in the C. acutatum complex, e.g. Glomerella acutata, a hybrid formed by crossing C. acutatum and C. fioriniae strains in the laboratory, and a putative hybrid strain between the same two species that had been collected from terminal crook disease on Pinus in New Zealand, where both species occur in nature (Damm et al. 2012a, this issue). Hybrids also form in the C. gloeosporioides complex, e.g. the Carya and Aeschynomene populations discussed by Cisar et al. (1994), more or less genetically equivalent to our species within the C. gloeosporioides complex.
Fig. 5.
A Bayesian inference phylogenetic tree of 30 selected isolates in the Kahawae clade of the Colletotrichum gloeosporioides species complex. The tree was build using concatenated sequences of the ACT, TUB2, CAL, CHS-1, GAPDH, GS, ITS, and SOD2 genes each with a separate model of DNA evolution. Other details as per Fig.1. B. A species-tree constructed from the same data, the scale is a clock set relative to the last common ancestor of the Kahawae clade and C. gloeosporioides s. str., as calibrated in Fig. 3.
Our taxonomic conclusions are based, of necessity, on the limited set of genes sampled. Potentially more powerful genes, such as ApMAT and Apn25L (Silva et al. 2012a) may provide finer resolution within the species-level clades that we recognise. However, even with these potentially more informative genes, the low levels of genetic divergence across the C. gloeosporioides complex may always provide a technical challenge (Silva et al. 2012a). The low level of diversity within this species complex is reflected by the branch lengths in fig. 2, Cannon et al. (2012, this volume), and is especially true across the Musae clade, where average pairwise identity between all isolates treated in our 5 gene alignment is 98.6 %. Pairwise identity between isolates of C. siamense and C. theobromicola, two species showing strong within-species phylogenetic structure, are 99.4 % and 99.6% respectively. This suggests that the species recognised within the C. gloeosporioides complex are very recently evolved and Silva et al. (2012b) provide data supporting this. Their hypothesis of recent evolution of host-specialised Colletotrichum populations from more generalist fungi was also invoked in relation to the C. acutatum complex by Lardner et al. (1999) using the “episodic selection” framework of Brasier (1995).
Several of the species we accept contain isolates with divergent lifestyles, for example C. aotearoa, C. clidemiae, C. kahawae, and C. theobromicola. Each of these species includes isolates capable of causing specific diseases. In the case of C. kahawae, recent pathogenicity tests have shown that only some isolates are able to cause coffee berry disease (Silva et al. 2012a, Silva & Weir, unpubl. data) and that these isolates can be distinguished using GS sequences (this study), Apn25L and MAT1-2-1 (Silva et al. 2012b). Because of the well understood pathogenicity of isolates within C. kahawae, the biosecurity importance of coffee berry disease, and the ability to distinguish the disease-causing isolates using carefully selected genetic markers, we recognise the disease-causing isolates taxonomically at the subspecific level. Future study of the comparative pathogenicity of isolates within C. aotearoa, C. clidemiae, and C. theobromicola may reveal genetically distinct, host-specialised pathogenic populations within these species that future workers may also choose to recognise taxonomically.
The classification we accept here is deliberately taxonomically conservative, minimising nomenclatural changes. This reflects continuing uncertainty about sensible species limits within the C. gloeosporioides complex that relate to low levels of genetic divergence across the complex, gene selection, isolate selection, and a lack of understanding of the mechanisms driving species and population divergence amongst these fungi. For example, the two haplotype subgroups of C. fructicola are not distinguished taxonomically because collectively they form a monophyletic clade, both subgroups include sets of isolates with similar geographic and host diversity, and there is no practical need to distinguish them taxonomically.
Molecular tools are increasingly being used for day-to-day identification by biosecurity officers and plant pathology researchers, providing a need for both a taxonomy that closely reflects groups that are resolved genetically, as well as simple and reliable protocols for identifying those taxa. The internal transcribed spacer region (ITS) has been proposed as the official fungal barcoding gene (Schoch et al. 2012). Although ITS is useful at the species complex level, it does a poor job of resolving species within the C. gloeosporioides complex, resolving only 10 of 22 accepted species. This reflects the low number of base changes in the ITS region across the C. gloeosporioides complex; species often distinguished by only one or two base changes. In some cases, chance variation in the ITS sequence within or between species means that some species cannot be distinguished (Fig. 6). Examples of taxa with identical ITS sequences include C. clidemiae, C. tropicale, C. ti and some C. siamense isolates; C. fructicola and some C. siamense isolates; and C. alienum, C. aenigma and some C. siamense isolates.
Protein-coding genes and their introns often have more variation than ITS, and the need for secondary barcodes based on these kinds of genes has been discussed in relation to some groups of fungi (Fitzpatrick et al. 2006, Aguileta et al. 2008, Weir & Johnston 2011). Ideally, one of the seven protein coding genes that were used in this study could be proposed as a secondary barcode to obtain an accurate identification of species within the C. gloeosporioides complex. A preliminary analysis of the genes performance as barcodes was conducted as part of Cai et al. (2009) with GAPDH, CAL, and ACT performing well, but CHS-1, ITS, and TEF (EF1α) poorly. However, the analysis (Cai et al. 2009) included only five species within the C. gloeosporioides complex, the Musae and Kahawae clades being treated at the level of species. With the final classification presented here, none of the genes we analysed provides an effective barcode ont its own across the entire complex. Of the single genes, TUB2, GS, and GAPDH are amongst the most effective at distinguishing species. However, C. clidemiae is polyphyletic in the TUB2 gene tree and GS sequences are needed to distinguish C. fructicola and C. alienum. With GS, C. aotearoa, C. kahawae subsp. ciggaro, and C. siamense are paraphyletic. GAPDH is the easiest of all the genes tested to amplify and sequence, however when using this gene GS sequences are needed to distinguish C. fructicola from C. alienum and C. aeschynomenes from C. siamense, and C. tropicale is paraphyletic. In the species descriptions we provide notes on which genes are the best for genetic identifications, and in Table 4 these are summarised for all species and genes. For species represented by a single or only a few isolates the species boundaries may not be accurate, we recommend two protein-coding genes in addition to ITS for sequence-based identifications. A meta-analysis of DNA barcodes across the whole genus will be required to find the combination of genes that are effective for all species of the genus that distinguish all Colletotrichum species.
Table 4.
Performance of individual genes at resolving species within the Colletotrichum gloeosporioides species complex. Y – species distinguished from all others. N – species not distinguished from all others. N* – distinguishes at the subspecies level.
| Species | ITS | GAPDH | CAL | TUB2 | ACT | CHS-1 | GS | SOD |
|---|---|---|---|---|---|---|---|---|
| C. fructicola | N | N | Y | N | N | Y | Y | Y |
| C. nupharicola | N | Y | Y | Y | Y | Y | Y | Y |
| C. alienum | N | N | Y | N | N | Y | Y | Y |
| C. musae | Y | Y | Y | Y | Y | Y | Y | Y |
| C. aenigma | N | Y | Y | Y | N | Y | Y | Y |
| C. siamense | N | N | Y | Y | N | N | N | N |
| C. aeschynomenes | N | N | N | Y | N | Y | Y | Y |
| C. tropicale | N | N | N | Y | Y | N | Y | Y |
| C. queenslandicum | N | Y | Y | Y | N | N | Y | N |
| C. salsolae | N | Y | Y | Y | Y | Y | N | Y |
| C. asianum | Y | Y | Y | Y | Y | Y | Y | Y |
| C. gloeosporioides | Y | Y | Y | Y | Y | Y | Y | Y |
| C. alatae | Y | Y | Y | Y | Y | Y | Y | Y |
| C. theobromicola | Y | Y | Y | Y | Y | Y | Y | Y |
| C. xanthorrhoeae | Y | Y | Y | Y | Y | Y | Y | Y |
| C. horii | Y | Y | Y | Y | Y | Y | Y | Y |
| C. aotearoa | N | N | Y | Y | N | Y | Y | N |
| C. ti | N | Y | Y | Y | N | Y | Y | Y |
| C. kahawae | N | Y | N | Y | Y | N | N* | N |
| G. cingulata “f. sp. camelliae” | Y | N | Y | Y | Y | N | Y | Y |
| C. clidemiae | N | N | N | N | Y | Y | Y | N |
| C. psidii | Y | Y | Y | Y | N | Y | Y | Y |
| C. cordylinicola | Y | Y | Y | Y | N | Y | Y | Y |
Several studies have shown that cultural morphology can be useful for grouping isolates when they are sampled at a local or regional level (e.g. Johnston & Jones 1997, Prihastuti et al. 2009). However, our experience is that such groups often break down when the geographic sample within a clade is extended to a global scale. Many of the species we accept have few or no diagnostic morphological or cultural features that can be consistently and reliably used to identify them. Our morphological examinations were confined to cultures on Difco PDA agar plates, and we will have missed any features that develop solely in association with plant material. In addition, the cultures we used have been sourced from different labs and collections from around the world, many with no information on storage history. Storage history and method has a major impact on the appearance of Colletotrichum in culture. Cultures can become “stale” during storage, losing the ability to produce pigments, the aerial mycelium often becoming very dense and felted, and losing the ability to form well-differentiated acervuli, conidia, or perithecia. In some clades, even freshly isolated cultures are highly variable, forming distinct sectors with differences in the production of pigment, aerial mycelium, acervuli, and conidia. Some isolates form two very different cultural types from single conidia or ascospores derived from colonies themselves started from single ascospores. Figure 27F shows single ascospore cultures from an isolate of C. kahawae subsp. ciggaro. One has the typical appearance of cultures of this fungus isolated from the field. The other, with a uniform, dense layer of conidia across the colony surface without well differentiated acervuli and more or less no aerial mycelium, is common from single ascospore isolates in culture, but rarely found in cultures isolated directly from the field. This kind of variation, and that revealed from sectoring during colony growth, makes morphological variation difficult to interpret for accurate identification.
Many of the species recognised in this work remain poorly understood in terms of their pathogenicity and host preference. This in part reflects a lack of certainty about the biological relationship between the fungi and the plants from which they were isolated. Species that are pathogenic on one host can also be isolated from others following opportunistic colonisation of senescing tissue, such as the C. salicis example discussed by Johnston (2000, as Glomerella miyabeana). The multiple Colletotrichum spp. associated with a single host are likely to have a variety of life styles: primary pathogens of healthy tissue, species with the ability to invade and cause minor disease when the host plant is under stress, species that develop latent infections and fruit only following senescence of the host tissue or ripening of host fruit and endophytic species that sporulate only following host tissue death. The combination of this range of distinct life styles, the fact that several Colletotrichum spp. may become established on a single host, and the ability of most of these species to also establish on a range of other hosts, has been a large part of the confusion surrounding species limits within Colletotrichum.
In some cases, apparently clear differences in pathogenicity of isolates in the C. gloeosporioides complex are not reflected genetically. For example, the fungi referred to as C. gloeosporioides f. stylosanthis “f. sp. guianensis” and C. gloeosporioides f. stylosanthis “f. sp. stylosanthis”, are reportedly associated with two distinct diseases of Stylosanthes (Irwin & Cameron 1978; Munaut et al. 2002), but both taxa genetically match C. theobromicola and are here placed in synonymy with C. theobromicola. It is possible that screening additional genes across a set of isolates from Stylosanthes with known pathogenicity will reveal one or more genes that generate a phylogeny that correlates with pathogenicity. This is the case with another specialised pathogen, C. kahawae. Originally described as a pathogen of green coffee berries, almost genetically identical isolates have subsequently been found on a wide range of hosts (see notes under C. kahawae). The isolates from other hosts are not pathogenic to coffee berries (Silva et al. 2012b). The difference in pathogenicity correlates with a genetic difference in the GS gene, and we taxonomically recognise this biologically specialised population at the subspecies level. A similar approach could potentially be taken for other biologically distinct populations within a genetically strongly supported species.
Despite the epitypification of C. gloeosporioides in 2008, web search hits on the name C. gloeosporioides from papers published over the past 12 mo show that many authors will continue to use the name in the sense of the C. gloeosporioides species complex, presumably regarding this level of identification as sufficient for their research. All of the isolates that we accept in the C. gloeosporioides complex share the string 5’–GGGCGGGT–3’ about 139–142 bases after the ITS1F primer binding site. Based on a comparison with GenBank data, this string appears to be specific to isolates that we would accept as members of the C. gloeosporioides complex. Several authors have developed PCR-based, rapid identification tools for distinguishing members of the C. gloeosporioides complex from members of the C. acutatum species complex. This has been prompted because some members of the C. acutatum complex have conidia without the acute ends characteristic of this species as described by Simmonds (1965), and have at times been confused with C. gloeosporioides (Damm et al. 2012, this issue). Primers reportedly specific to C. gloeosporioides include the CgInt primer for ITS (Mills et al. 1992). In our data set this primer sequence is found in C. gloeosporioides s. str., C. fructicola, and C. siamense but all of the other taxa that we recognise within the C. gloeosporioides complex have one or more bases not matching the CgInt primer. The practical impact of these differences will depend in part on the position of the mismatch and stringency of the PCR reaction. Talhinas et al. (2005) discussed the TBCG primer for β-tubulin, and this is found within all of our taxa within the C. gloeosporioides group except C. musae and C. asianum. Liu et al. (2011) describe characteristic RFLP bands from glutamine synthetase using the restriction enzyme Pst1. Based on our sequences, this method will generate the characteristic C. gloeosporioides bands reported by Liu et al. (2011) for C. aenigma, C. alienum, C. aotearoa, C. asianum, C. clidemiae, C. cordylinicola, C. fructicola, C. gloeosporioides s. str., C. horii, C. queenslandicum, C. salsolae, C. siamense, C. ti, and C. tropicale. Different banding patterns will be produced by C. aeschynomenes (band sizes 253, 316, 388), the two C. kahawae subspp. (band sizes 112, 388, 457), G. cingulata “f. sp. camelliae” (band sizes 51, 112, 337, 457), and C. musae (band sizes 388, 552), but none match the bands reported for C. acutatum by these authors.
Comparison of our data with gene sequences reported as C. gloeosporioides in recent papers allows most to be placed with confidence in one of the species that we accept. There are exceptions, such as the pecan-associated isolates from Liu et al. (2011), and the pistachio-associated isolates reported by Yang et al. (2011), both of which appear to represent undescribed species within the C. gloeosporioides complex. Clearly, more species remain to be described within the C. gloeosporioides complex. In addition, taxonomic issues still to be resolved amongst the species discussed in this paper include the relationship between G. cingulata “f. sp. camelliae” and C. camelliae, the identity of the cotton pathogens referred to C. gossypii, the identity of the cassava pathogens referred to C. manihotis, the relationship between C. aeschynomenes and C. gloeosporioides “f. sp. jussiaeae”, whether the various yam diseases discussed in the literature are all caused by C. alatae, and whether the isolates of C. aotearoa from Meryta leaf spots form a biologically distinct population. A more general question relates to better understanding the frequency of hybrids within the C. gloeosporioides complex and the impact of this on the interpretation of the phylogenies within the complex. The impact of hybridisation on the evolution of disease specialised populations has barely been explored.
Acknowledgments
This study was funded by AGMARDT (The Agricultural and Marketing Research and Development Trust) grant no. 892, the New Zealand Ministry of Science and Innovation through backbone funding of the “Defining New Zealand’s Land Biota” programme, and the New Zealand Tertiary Education Commission through the “Molecular diagnostics: capitalising on a million DNA barcodes” project. Shaun Pennycook, Landcare Research, provided valuable nomenclatural advice, Duckchul Park and Paula Wilkie, Landcare Research, provided valuable technical assistance. Diogo Silva, Centro de Investigação das Ferrugens do Cafeeiro, Portugal, carried out the C. kahawae pathogenicity tests. David Cleland, ATCC, provided DNA sequences for ATCC 96723. This study has been possible only through the provision of cultures by the DAR, ICMP, CBS, CABI, and MAFF culture collections, as well as by many individual researchers — Matthew Abang, Deutsche Sammlung von Mikroorganismen und Zellkulturen, Braunschweig, Germany; Robert Barreto, Universidade Federal de Viçosa, Brazil; George Carroll, University of Oregon, USA; Dana Berner, Foreign Disease-Weed Science Research Unit, USDA-ARS, Ft. Detrick, USA; Lindy Coates, Brad McNeil and Roger Shivas, Queensland Primary Industries and Fisheries, Indooroopilly, Queensland, Australia; W.E. Copes, USDA-ARS Small Fruit Research Unit, Poplarville, Massachusetts, USA; Kerry Everett and Mike Manning, Plant and Food Research, Auckland, New Zealand; David Farr, Gary Samuels and Steve Rehner, Systematic Mycology & Microbiology Laboratory USDA, Beltsville, USA; Steve Ferriera and K. Pitz, University of Hawaii, USA; Stan Freeman, Dept. of Plant Pathology and Weed Research, The Volcani Center, Israel; Hideo Ishii, National Institute for Agro-Environmental Sciences, Tsukuba, Japan; Sucheta Joshee and Nick Waipara, Landcare Research, Auckland, New Zealand; Lisa Korsten, University of Pretoria, South Africa; Lei Cai, State Key Laboratory of Mycology, Institute of Microbiology, Chinese Academy of Sciences, Beijing, China; Frank Louws and Turner Sutton, North Carolina State University, USA; Stephen Mackenzie and Natalia Peres, University of Florida, USA; Nelson Massola, Escola Superior de Agricultura “Luiz de Queiroz”, Brazil; Jean-Yves Meyer, Department of Research Ministère d’Education, de l’Enseignement Supérieur et de la Recherche Gouvernement de Polynésie française, French Polynesia; François Munaut, Université Catholique de Louvain, Belgium; Vera Sergeeva, University of Western Sydney, New South Wales, Australia; Amir Sharon, Tel Aviv University, Israel; Po Po Than, Chinese Academy of Forestry, Kunming, China; Ramon Villanueva-Aroe, University of València, Spain; Jing-Ze Zhang, Zhejiang University, Hangzhou, China.
REFERENCES
- Aa HA van der. (1978). A leaf spot of Nymphaea alba in the Netherlands. Netherlands Journal of Plant Pathology 84: 109–115 [Google Scholar]
- Abang MM, Winter S, Green KR, Hoffmann P, Mignouna HD, Wolf GA. (2002). Molecular identification of Colletotrichum gloeosporioides causing anthracnose of yam in Nigeria. Plant Pathology 51: 63–71 [Google Scholar]
- Abang MM, Winter S, Mignouna HD, Green KR, Asiedu R. (2003). Molecular taxonomic, epidemiological and population genetic approaches to understanding yam anthracnose disease. African Journal of Biotechnology 2: 486–496 [Google Scholar]
- Afanador-Kafuri L, Minz D, Maymon M, Freeman S. (2003). Characterisation of Colletotrichum isolates from tamarillo, passiflora, and mango in Colombia and identification of a unique species from the genus. Phytopathology 93: 579–587 [DOI] [PubMed] [Google Scholar]
- Aguileta G, Marthey S, Chiapello H, Lebrun MH, Rodolphe F, et al. (2008). Assessing the Performance of Single-Copy Genes for Recovering Robust Phylogenies Systematic Biology 57: 613–627 [DOI] [PubMed] [Google Scholar]
- Alahakoon PW, Brown AE, Sreenivasaprasad S. (1994). Genetic characterisation of Colletotrichum gloeosporioides isolates obtained from mango. International Journal of Pest Management 40: 225–229 [Google Scholar]
- Alfieri SA, Jr, Langdon KR, Wehlburg C, Kimbrough JW. (1984). Index of Plant Diseases in Florida (Revised). Florida Department of Agriculture and Consumer Services, Division of Plant Industries Bulletin 11: 1–389 [Google Scholar]
- Archibald JK, Mort ME, Crawford DJ. (2003). Bayesian inference of phylogeny: a non-technical primer. Taxon 52: 187–191 [Google Scholar]
- Arx JA von. (1957). Die Arten der Gattung Colletotrichum Cda. Phytopathologische Zeitschrift 29: 413–468 [Google Scholar]
- Arx JA von. (1970). A revision of the fungi classified as Gloeosporium. Bibliotheca Mycologica 24: 1–203 [Google Scholar]
- Arx JA von, Müller E. (1954). Die Gattung der amerosporen Pyrenomyceten. Beiträge zur Kryptogamenflora der Schweiz 11(1): 1–434 [Google Scholar]
- Bailey JA, Nash C, Morgan LW, O’Connell RJ, TeBeest DO. (1996). Molecular taxonomy of Colletotrichum species causing anthracnose on the Malvaceae. Phytopathology 86: 1076–1083 [Google Scholar]
- Beever RE. (1984). Observations on puka (Meryta sinclairii) on the Chickens Islands, with an assessment of the hypothesis that it was transferred there by the Maori. Tane 30: 77–92 [Google Scholar]
- Berkeley MJ. (1874). Notices of North American fungi. Grevillea 3: 1–17 [Google Scholar]
- Berner DK, Bruckart WL, Cavin CA, Michael JL, Carter ML, Luster DG. (2009). Best linear unbiased prediction of host-range of the facultative parasite Colletotrichum gloeosporioides f. sp. salsolae, a potential biological control agent of Russian thistle. Biological Control 51: 158–168 [Google Scholar]
- Bogo A, Casa RT, Rufato L, Gonçalves MJ. (2012). The effect of hail protection nets on Glomerella leaf spot in ‘royal Gala’ apple. Crop Protection 31: 40–44 [Google Scholar]
- Boyette CD, Templeton GE, Smith JR. (1979). Control of waterprimrose (Jussiaea decurrens) and northern jointvetch (Aeschynomene virginica) with fungal pathogens. Weed Science 27: 497–501 [Google Scholar]
- Brasier CM. (1995). Episodic selection as a force in fungal microevolution, with special reference to clonal speciation and hybrid introgression. Canadian Journal of Botany 73 (Supplement 1): S1213–S1221 [Google Scholar]
- Brooks AN. (1931). Anthracnose of strawberry caused by Colletotrichum fragariae n. sp. Phytopathology 21: 739–744 [Google Scholar]
- Buddie AG, Martínez-Culebras P, Bridge PD, García MD, Querol A, et al. (1999). Molecular characterisation of Colletotrichum strains derived from strawberry. Mycological Research 103: 385–394 [Google Scholar]
- Cai L, Hyde KD, Taylor PWJ, Weir BS, Waller J, et al. (2009). A polyphasic approach for studying Colletotrichum. Fungal Diversity 39: 183–204 [Google Scholar]
- Cannon PF, Buddie AG, Bridge PD. (2008). The typification of Colletotrichum gloeosporioides. Mycotaxon 104: 189–204 [Google Scholar]
- Cannon PF, Damm U, Johnston PR, Weir BS. (2012). Colletotrichum – current status and future directions. Studies in Mycology 73: 181–213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carbone I, Kohn LM. (1999). A method for designing primer sets for speciation studies in filamentous ascomycetes. Mycologia 91: 553–556 [Google Scholar]
- Carpenter JB, Stevenson JA. (1954). A secondary leaf spot of the Hevea rubber tree caused by Glomerella cingulata. Plant Disease Reporter 38: 494–499 [Google Scholar]
- Castro RA, Mendes-Costa MC, Souza EA. (2006). Dimorfismo de ascósporos em Glomerella cingulata f. sp. phaseoli. Fitopatologia Brasileira 31: 598–600 [Google Scholar]
- Chevaugeon J. (1956). Les maladies cryptogamiques du manioc en Afrique Occidentale. Encyclopédie Mycologique 28: 1–205 [Google Scholar]
- Chung WS, Ishii H, Nishimura K, Fukaya M, Yano K, Kajitani Y. (2006). Fungicide sensitivity and phylogenetic relationship of anthracnose fungi isolated from various fruit crops in Japan. Plant Disease 90: 506–512 [DOI] [PubMed] [Google Scholar]
- Chung Y, Ané C. (2011). Comparing Two Bayesian Methods for Gene Tree/Species Tree Reconstruction: Simulations with Incomplete Lineage Sorting and Horizontal Gene Transfer. Systematic Biology 60: 261–275 [DOI] [PubMed] [Google Scholar]
- Cisar CR, Spiegel FW, TeBeest DO, Trout C. (1994). Evidence for mating between isolates of Colletotrichum gloeosporioides with different host specificities. Current Genetics 25: 330–335 [DOI] [PubMed] [Google Scholar]
- Correll JC, Rhoads DD, Guerber JC. (1993). Examination of mitochondrial DNA restriction fragment length polymophisms, DNA fingerprints, and randomly amplified polymorphic DNA of Colletotrichum orbiculare. Phytopathology 83: 1199–1204 [Google Scholar]
- Crouch JA, Clarke BB, Hillman BI. (2006). Unravelling evolutionary relationships amongst divergent lineages of Colletotrichum causing anthracnose disease in turfgrass and corn. Phytopathology 96: 46–60 [DOI] [PubMed] [Google Scholar]
- Crous PW, Gams W, Stalpers JA, Robert V, Stegehuis G. (2004). MycoBank: an online initiative to launch mycology into the 21st century. Studies in Mycology 50: 19–22 [Google Scholar]
- Curzi M. (1927). De novis Eumycetibus. Atti dell’Istituto Botanico della Università e Laboratorio Crittogamico di Pavia 3 (3): 203–208 [Google Scholar]
- Damm U, Cannon PF, Woudenberg JHC, Crous PW. (2012a). The Colletotrichum acutatum species complex. Studies in Mycology 73: 37–113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damm U, Cannon PF, Woudenberg JHC, Johnston PR, Weir BS, et al. (2012b). The Colletotrichum boninense species complex. Studies in Mycology 73: 1–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniel JT, Templeton GE, Smith RJ, Fox WT. (1973). Biological control of northern jointvetch in rice with an endemic fungal disease. Weed Science 21: 303–307 [Google Scholar]
- Delacroix G. (1903). De la tavelure goyaves produite par le Gloeosporium psidii nov. sp. G. Del. Bulletin de la Société Mycologique de France 19: 143–145 [Google Scholar]
- Deng Y, Zhang M, Luo H. (2012). Identification and antimicrobial activity of two alkaloids from traditional Chinese medicinal plant Tinospora capillipes. Industrial Crops and Products 37: 298–302 [Google Scholar]
- Dickens JSW, Cook RTA. (1989). Glomerella cingulata on Camellia. Plant Pathology 38: 75–85 [Google Scholar]
- Ditmore M, Moore JW, TeBeest DO. (2008). Interactions of two selected field isolates of Colletotrichum gloeosporioides f. sp. aeschynomene on Aeschynomene virginica. Biological Control 47: 298–308 [Google Scholar]
- Drummond AJ, Ashton B, Buxton S, Cheung M, Cooper A, et al. (2011). Geneious v5.4, Available from http://www.geneious.com/
- Drummond AJ, Suchard MA, Xie D, Rambaut A. (2012). Bayesian phylogenetics with BEAUti and the BEAST 1.7. Molecular Biology and Evolution 29: 1969–1973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du M, Schardl CL, Nuckles EM, Vaillancourt L. (2005). Using mating-type gene sequences for improved phylogenetic resolution of Colletotrichum species complexes. Mycologia 97: 641–658 [DOI] [PubMed] [Google Scholar]
- Edgerton CW. (1909). The perfect stage of the cotton anthracnose. Mycologia 1: 115–120 [Google Scholar]
- Farr DF, Aime MC, Rossman AY, Palm ME. (2006). Species of Colletotrichum on Agavaceae. Mycological Research 110: 1395–1408 [DOI] [PubMed] [Google Scholar]
- Fitzpatrick DA, Logue ME, Stajich JE, Butler G. (2006). A fungal phylogeny based on 42 complete genomes derived from supertree and combined gene analysis. BMC Evolutionary Biology 6: 99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fokunang CN, Dixon AGO, Akem CN, Ikotun T. (2000). Cutural, morphological and pathogenic variability in Colletotrichum gloeosporioides f. sp. manihotis isolates from cassava (Manihot esculenta) in Nigeria. Pakistan Journal of Biological Sciences 3: 542–546 [Google Scholar]
- Gardes M, Bruns TD. (1993). ITS primers with enhanced specificity for basidiomycetes – application to the identification of mycorrhizae and rusts. Molecular Ecology 2: 113–118 [DOI] [PubMed] [Google Scholar]
- Glass NL, Donaldson GC. (1995). Development of primer sets designed for use with the PCR to amplify conserved genes from filamentous ascomycetes. Applied and Environmental Microbiology. 61: 1323–1330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gielink AJ, Vermeulen H. (1983). The ability of American and African Colletotrichum isolates to cause coffee berry disease symptoms and the association of some isolates with Glomerella cingulata. Netherlands Journal of Plant Pathology 89: 188–190 [Google Scholar]
- Gorter GJMA. (1956). Anthracnose fungi of olives. Nature 178: 1129–1130 [Google Scholar]
- Green KR, Simons SA. (1994). ‘Dead skin’ on yams (Dioscorea alata) caused by Colletotrichum gloeosporioides. Plant Pathology 43: 1062–1065 [Google Scholar]
- Greene GL. (1967). Effect of 2-dehydroxy-D-glocose on the respiration of tropical anthracnose fungi. Physiologia Plantarum 20: 580–586 [Google Scholar]
- Guerber JC, Correll JC. (2001). Characterisation of Glomerella acutata, the teleomorph of Colletotrichum acutatum. Mycologia 93: 216–229 [Google Scholar]
- Guerber JC, Liu B, Correll JC, Johnston PR. (2003). Characterization of diversity in Colletotrichum acutatum sensu lato by sequence analysis of two gene introns, mtDNA and intron RFLPs, and mating compatibility. Mycologia 95: 872–895 [PubMed] [Google Scholar]
- Gunnell PS, Gubler WD. (1992). Taxonomy and morphology of Colletotrichum species pathogenic to strawberry. Mycologia 84: 157–165 [Google Scholar]
- Harris P. (1993). Effects, constraints and the future of weed biocontrol. Agriculture, Ecosystems and Environment 46: 289–303 [Google Scholar]
- Heled J, Drummond AJ. (2010). Bayesian Inference of Species Trees from Multilocus Data. Molecular Biology and Evolution 27: 570–580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins BB. (1926). Anthracnose of pepper (Capsicum anuum L.). Phytopathology 16: 333–345 [Google Scholar]
- Hildebrand PD, Jensen KIN. (1991). Potential for the biological control of St Johns’s-wort (Hypericum perforatum) with an endemic strain of Colletotrichum gloeosporioides. Canadian Journal of Plant Pathology 13: 60–70 [Google Scholar]
- Hindorf H. (1973). Colletotrichum-Population auf Coffea arabica L. in Kenia II. Qualitative und quantitative Unterschiede in der Colletotrichum-Population. Phytopathologische Zeitschrift 77: 216–234 [Google Scholar]
- Hosdson A, Mills PR, Brown AE. (1993). Ribosomal and mitochondrial DNA polymorphisms in Colletotrichum gloeosporioides isolated from tropical fruits. Mycological Research 97: 329–335 [Google Scholar]
- Hsiang T, Goodwin PH. (2001). Ribosomal DNA sequence comparisons of Colletotrichum graminicola from turfgrasses and other hosts. European Journal of Plant Pathology 107: 593–599 [Google Scholar]
- Hyde KD, Cai L, Cannon PF, Crouch JA, Crous PW, et al. (2009). Colletotrichum – names in current use. Fungal Diversity 39: 147–182 [Google Scholar]
- Hyde KD, Chomnunti P, Crous PW, Groenewald JZ, Damm U, et al. (2010). A case for re-inventory of Australia’s plant pathogens. Persoonia 25: 50–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irwin JAG, Cameron DF. (1978). Two diseases in Stylosanthes caused by Colletotrichum gloeosporioides in Australia, and pathogenic specialisation within one of the causal organisms. Australian Journal of Agricultural Research 29: 3015–317 [Google Scholar]
- Johnson DA, Carris LM, Rogers JD. (1997). Morphological and molecular characterization of Colletotrichum nymphaeae and C. nupharicola sp. nov. on water-lilies (Nymphaea and Nuphar). Mycological Research 101: 641–649 [Google Scholar]
- Johnston PR. (2000). The importance of phylogeny in understanding host relationships within Colletotrichum. In: Colletotrichum: host specificity, pathogenicity, and host-pathogen interactions (Prusky D, Dickman MB, Freeman S. eds). APS Press, St Paul, Minnesota: 21–28 [Google Scholar]
- Johnston PR, Jones D. (1997). Relationships among Colletotrichum isolates from fruit rots assessed using rDNA sequences. Mycologia 89: 420–430 [Google Scholar]
- Johnston PR, Pennycook SR, Manning MA. (2005). Taxonomy of fruit-rotting fungal pathogens: what’s really out there? New Zealand Plant Protection 58: 42–46 [Google Scholar]
- Kaan F. (1973). A study on inheritance of eggplant (Solanum melongena L.) resistance to fruit anthracnose (Colletotrichum gloeosporioides f. sp. melongenae Penzig Fournet). Annales De l’Amelioration des Plantes 23:127–151 [Google Scholar]
- Kenny MK, Galea VJ, Price TV. (2012). Germination and growth of Colletotrichum acutatum and Colletotrichum gloeosporioides isolates from coffee in Papua New Guinea and their pathogenicity. Australasian Plant Pathology (online, doi:10.1007/s13313-012-0117-7).
- Killgore EM, Sugiyama LS, Barreto RW, Gardner DE. (1999). Evaluation of Colletotrichum gloeosporioides for Biological Control of Miconia calvescens in Hawaii. Plant Disease 83: 964 [DOI] [PubMed] [Google Scholar]
- Kornerup A, Wanscher JH. (1963). Methuen handbook of colour. Methuen and Co. London: [Google Scholar]
- Krauss U, Matthews P, Bidwell R, Hocart M, Anthony F. (2001). Strain discrimination by fungal antagonists of Colletotrichum musae: implications for biocontrol of crown rot of banana. Mycological Research 105: 67–76 [Google Scholar]
- Lardner R, Johnston PR, Plummer KM, Pearson MN. (1999). Morphological and molecular analysis of Colletotrichum acutatum sensu lato. Mycological Research 103: 275–285 [Google Scholar]
- Larkin MA, Blackshields G, Brown NP, Chenna R, McGettigan PA, et al. (2007). Clustal W and Clustal X v. 2.0. Bioinformatics 23: 2947–2948 [DOI] [PubMed] [Google Scholar]
- Laundon GF. (1972) [1971] Records of fungal plant diseases in New Zealand – 2. New Zealand Journal of Botany 9: 610–624 [Google Scholar]
- Li R, Ma H. (1993). Studies in the occurrence and control of ramie anthracnose. Acta Phytophylacica Sinica 20: 83–89 [in Chinese] [Google Scholar]
- Liu LJ. (1972). Identification and occurrence of perfect stage and cultural and morpholoical variants of Colletotrichum gloeosporioides from guava in Puerto Rico. Journal of Agriculture of the University of Puerto Rico 56: 171–180 [Google Scholar]
- Liu B, Louws FJ, Sutton TB, Correll JC. (2011). A rapid qualitative molecular method for the identification of Colletotrichum acutatum and C. gloeosporioides. European Journal of Plant Pathology 132: 593–607 [Google Scholar]
- Lubbe CM, Denman S, Cannon PF, Groenewald JZ, Lamprecht SC, Crous PW. (2004). Characterisation of Colletotrichum species associated with Proteaceae. Mycologia 96: 1268–1279 [PubMed] [Google Scholar]
- MacCaughey V. (1917). The guavas of the Hawaiian Islands. Bulletin of the Torrey Botanical Club 44: 513–524 [Google Scholar]
- Mackenzie SJ, Seijo TE, Legard DE, Timmer LW, Peres NA. (2007). Selection for pathogenicity to strawberry in populations of Colletotrichum gloeosporioides from native plants. Phytopathology 97: 1130–1140 [DOI] [PubMed] [Google Scholar]
- Mackenzie SJ, Mertely JC, Seijo TE, Peres NA. (2008). Colletotrichum fragariae is a pathogen on hosts other than strawberry. Plant Disease 92: 1432–1438 [DOI] [PubMed] [Google Scholar]
- McNeill J, Barrie FR, Burdet HM, Demoulin V, Hawksworth DL, et al. (2006). International Code of Botanical Nomenclature (Vienna Code). Adopted by the Seventeenth International Botanical Congress, Vienna, Austria, July 2005 Regnum Vegetabile 146. 568 p [Google Scholar]
- Makambila C. (1994). The fungal diseases of cassava in the Republic of Congo, Central Africa. African Crop Science Journal 2: 511–517 [Google Scholar]
- Makowski RMD, Mortensen K. (1989). Colletotrichum gloeosporioides f. sp. malvae as a bioherbicide for round-leafed mallow (Malva pusilla): conditions for successful control in the field. In: Proceedings of the 7th International Symposium for the Biological Control of Weeds. (Delfosse ES. ed.). Istituto Sperimentale per la Patalogia Vegetale, Rome: 513–522 [Google Scholar]
- Marcelino J, Giordano R, Gouli S, Gouli V, Parker BL, et al. (2008). Colletotrichum acutatum var. fioriniae (teleomorph: Glomerella acutata var. fioriniae var. nov.) infection of a scale insect. Mycologia 100: 353–374 [DOI] [PubMed] [Google Scholar]
- Martínez-Culebras PV, Barrio E, García MD, Querol A. (2000). Identification of Colletotrichum species responsible for anthracnose of strawberry based on the internal transcribed spacers of the ribosomal region. FEMS Microbiology Letters 189: 97–101 [DOI] [PubMed] [Google Scholar]
- Martinez-Culebras PV, Querol A, Suarez-Fernandez MB, Garcia-Lopez MD, Barrio E. (2003). Phylogenetic relationships among Colletotrichum pathogens of strawberry and design of PCR primers for their identification. Journal of Phytopathology 151: 135–143 [Google Scholar]
- Maymon M, Zveibil A, Pivonia S, Minz D, Freeman S. (2006). Identification and characterisation of benomyl-resistant and sensitive-populations of Colletotrichum gloeosporioides from statice (Limonium spp.). Phytopathology 96: 542–548 [DOI] [PubMed] [Google Scholar]
- Menten JOM, Kimati H, Costa CP. (1980). Reação de populações de pepino (Cucumis sativus L.) a Colletotrichum gloeosporioides f. sp. cucurbitae (Berk. et Mont.) N. Comb. Summa Phytopathologica 5: 127–133 [Google Scholar]
- Mills PR, Sreenivasaprasad S, Brown AE. (1992). Detection and differentiation of Colletotrichum gl Maymon oeosporioides isolates using PCR. FEMS Microbiological Letters 98: 137–144 [DOI] [PubMed] [Google Scholar]
- Misra AK. (2004). Guava diseases – their symptoms, causes and management. In: Diseases of fruits and vegetables. Diagnosis and management Volume 2. (Naqvi SAMH. ed.). Kluwer Academic Publishers, Dordrecht: 81–119 [Google Scholar]
- Mordue JEM. (1971). Glomerella cingulata. CMI Desriptions of Plant Pathogenic Fungi No. 315 [Google Scholar]
- Commonwealth Mycological Institute, Kew Moriwaki J, Sato T, Tsukiboshi T. (2003). Morphological and molecular characterisation of Colletotrichum boninense sp. nov. from Japan. Mycoscience 44: 47–53 [Google Scholar]
- Moriwaki J, Sato T. (2009). A new combination for the causal agent of tea anthracnose: Discula theae-sinensis (I. Miyake) Moriwaki & Toy. Sato, comb. nov. Journal of General Plant Pathology 75: 359–361 [Google Scholar]
- Moriwaki J, Tsukiboshi T. (2009). Colletotrichum echinochloae, a new species on Japanese barnyard millet (Echinochloa utilis). Mycoscience 50: 273–280 [Google Scholar]
- Munaut F, Hamaide N, Maraite H. (2002). Genomic and pathogenic diversity in Colletotrichum gloeosporioides from wild native Mexican Stylosanthes spp., and taxonomic implications. Mycological Research 106: 579–593 [Google Scholar]
- Munaut F, Hamaide N, Vander Stappen J, Maraite H. (1998). Genetic relationships among isolates of Colletotrichum gloeosporioides from Stylosanthes spp. in Africa and Australia using RAPD and ribosomal DNA markers. Plant Pathology 47: 641–648 [Google Scholar]
- Muraleedharan N, Baby UI. (2007). Tea disease: ecology and control. In: Encyclopedia of Pest Management Vol. 2 (Pimentel D, ed.) [electronic resource] CRC Press, Boca Raton: 668–671 [Google Scholar]
- Nilsson RH, Ryberg M, Kristiansson E, Abarenkov K, Larsson KH, Kõljalg U. (2006). Taxonomic reliability of DNA sequences in public sequence databases: a fungal perspective. PLoS ONE 1(1): e59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nirenberg HI, Feiler U, Hagedorn G. (2002). Description of Colletotrichum lupini comb. nov. in modern terms. Mycologia 94: 307–320 [PubMed] [Google Scholar]
- Noak F. (1901). Die Krankheiten des Kaffeebaumes in Brasilien. Zeitschrift für Pflanzenkrankheiten 11: 196–203 [Google Scholar]
- Ocfemia GO, Agati JA. (1925). The cause of anthracnose of avocado, mango and upo in the Philippine Islands. Philippine Agriculturalist 14: 199–216 [Google Scholar]
- O’Donnell K, Cigelnik E. (1997). Two divergent intragenomic rDNA ITS2 types within a monophyletic lineage of the fungus Fusarium are nonorthologous. MoIecular Phylogenetics and Evolution 7:103–116 [DOI] [PubMed] [Google Scholar]
- O’Donnell K, Nirenberg HI, Aoki T, Cigelnik E. (2000). A Multigene phylogeny of the Gibberella fujikuroi species complex: Detection of additional phylogenetically distinct species. Mycoscience 41: 61–78 [Google Scholar]
- Owolade OF, Dixon AGO, Alabi BS, Akande SR, Olakojo SA. (2008). A combining ability analysis of cassava Manihot esculenta Crantz genotypes to anthracnose disease. Electronic Journal of Environmental, Agricultural and Food Chemistry 7: 2959–2968 [Google Scholar]
- Parvin S, Lee OR, Sathiyaraj G, Khorolragchaa A, Kim YJ, et al. (2012). Interrelationship between calmodulin (CaM) and H2O2 in abscisic acid-induced antioxidant defense in the seedlings of Panax ginseng. Molecular Biology Reports 39: 7327–7338 [DOI] [PubMed] [Google Scholar]
- Penzig AGO. (1882). Fungi agrumicoli. Contribuzione allo studio dei funghi parassiti degli agrumi. Michelia 2: 385–508 [Google Scholar]
- Phoulivong S, Cai L, Parinn N, Chen H, Abd-Elsalam K, et al. (2011). A new species of Colletotrichum from Cordyline fruticosa and Eugenia javanica causing anthracnose disease. Mycotaxon 114: 247–257 [Google Scholar]
- Polashock JJ, Caruso FL, Oudemans PV, McManus PS, Crouch JA. (2009). The North American cranberry fruit rot fungal community: a systematic overview using morphological and phylogenetic affinities. Plant Pathology 58: 1116–1127 [Google Scholar]
- Pollacci G. (1899). Contribuzione alla micologia ligustica. Atti dell’ Istituto Botanico dell’ Università di Pavia, Serie 2 5: 29–46 [Google Scholar]
- Posada D. (2008). jModelTest: Phylogenetic Model Averaging. Molecular Biology and Evolution 25: 1253–1256 [DOI] [PubMed] [Google Scholar]
- Prasad N, Singh RD. (1960). Anthracnose disease of Dioscorea alata L. (Yam, Eng., Ratalu, Hind.). Current Science 29: 66–67 [Google Scholar]
- Prihastuti H, Cai L, Chen H, McKenzie EHC, Hyde KD. (2009). Characterisation of Colletotrichum species associated with coffee berries in northern Thailand. Fungal Diversity 39: 89–109 [Google Scholar]
- R Development Core Team (2011). R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria: [Google Scholar]
- Rambaut A, Drummond AJ. (2007). Tracer v. 1.4, Available from http://beast.bio.ed.ac.uk/Tracer
- Rodrigues CJ, Varzea VMP, Hindorf H, Medeiros EF. (1991). Strains of Colletotrichum coffeanum Noack causing coffee berry disease in Angola and Malawi with characteristics different to the Kenya strain. Journal of Phytopathology 131: 205–209 [Google Scholar]
- Rodriguez RJ, Owen JL. (1992). Isolation of Glomerella musae [teleomorph of Colletotrichum musae (Berk. & Curt.) Arx] and segregation analysis of ascospore progeny. Experimental Mycology 16: 291–301 [Google Scholar]
- Rodríguez-Guerra R, Ramírez-Rueda M, Cabral-Enciso M, García-Serrano M, Lira-Maldonado Z, et al. (2005). Heterothallic mating observed between Mexican isolates of Glomerella lindemuthiana. Mycologia 97: 793–803 [DOI] [PubMed] [Google Scholar]
- Rojas EI, Rehner SA, Samuels GJ, Van Bael SA, Herre EA, et al. (2010). Colletotrichum gloeosporioides s.l. associated with Theobroma cacao and other plants in Panamá: multilocus phylogenies distinguish host-associated pathogens from asymptomatic endophytes. Mycologia 102: 1318–1338 [DOI] [PubMed] [Google Scholar]
- Ronquist F, Teslenko M, van der Mark P, Ayres D, Darling A, et al. (2012). MrBayes v. 3.2: Efficient Bayesian phylogenetic inference and model choice across a large model space. Systematic Biology 61: 539–542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saccardo PA, Trotter A. (1913). Supplementum Universale Pars. IX. Ascomycetae-Deuteromycetae. Sylloge Fungorum 22: 1–1612 [Google Scholar]
- Sanders GM, Korsten L. (2003). Comparison of cross inoculation potential of South African avocado and mango isolates of Colletotrichum gloeosporioides. Microbiological Research 158: 143–150 [DOI] [PubMed] [Google Scholar]
- Sawada T. (1922). New Japanese fungi notes and translations – XI. Mycologia 14: 81–89 [Google Scholar]
- Schoch CL, Seifert KA, Huhndorf S, Robert V, Spouge JL, et al. (2012). Nuclear ribosomal internal transcribed spacer (ITS) region as a universal DNA barcode marker for Fungi. Proceedings of the National Academy of Sciences 109: 6241–6246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarczinger I, Vajna L, Bruckart WL. (1998). First report of Colletotrichum gloeosporioides on Russian thistle. Plant Disease 82: 1405 [DOI] [PubMed] [Google Scholar]
- Seifert KA. (2009). Progress towards DNA barcoding of fungi. Molecular Ecology Resources 9 (Suppl. 1): 83–89 [DOI] [PubMed] [Google Scholar]
- Shear CL. (1907). New species of fungi. Bulletin of the Torrey Botanical Club 34: 305–317 [Google Scholar]
- Shear CL, Wood AK. (1907). Ascogenous forms of Gloeosporium and Colletotrichum. Botanical Gazette 43: 259–266 [Google Scholar]
- Shear CL, Wood AK. (1913). Studies of fungus parasites belonging to the genus Glomerella. USDA Bureau of Plant Industry Bulletin 252: 1–110 [Google Scholar]
- Sheldon JL. (1906). The ripe rot or mummy disease of guavas. Bulletin of the West Virginia University Experimental Station 104: 299–315 [Google Scholar]
- Shenoy BD, Jeewon R, Lam WH, Bhat DJ, Than PP, et al. (2007). Morpho-molecular characterisation and epitypification of Colletotrichum capsici (Glomerellaceae, Sordariomycetes), the causative agent of anthracnose on chilli. Fungal Diversity 27: 197–211 [Google Scholar]
- Sherf AF, MacNab AA. (1986). Vegetable diseases and their control. John Wiley and Sons; USA: [Google Scholar]
- Sherriff C, Whelan MJ, Arnold GM, Lafay JF, Brygoo Y, Bailey JA. (1994). Ribosomal DNA sequence analysis reveals new species groupings in the genus Colletotrichum. Experimental Mycology 18: 121–138 [Google Scholar]
- Shivas RG, Bathgate J, Podger FD. (1998). Colletotrichum xanthorrhoeae sp. nov. on Xanthorrhoea in Western Australia. Mycological Research 102: 280–282 [Google Scholar]
- Silva DN, Talhinas P, Várzea V, Cai L, Paulo OS, Batista D. (2012a). Application of the Apn2/MAT locus to improve the systematics of the Colletotrichum gloeosporioides complex: an example from coffee (Coffea spp.) hosts. Mycologia 104: 396–409 [DOI] [PubMed] [Google Scholar]
- Silva DN, Talhinhas P, Cai L, Manuel L, Gichuru EK, et al. (2012b). Host-jump drives rapid and recent ecological speciation of the emergent fungal pathogen Colletotrichum kahawae. Molecular Ecology 21: 2655–2670 [DOI] [PubMed] [Google Scholar]
- Silva VN da, Guzzo SD, Mantovanello-Lucon CM, Harakava R. (2011). Promoção de crescimento e inducção de resistência à antracnose por Trichoderma spp. em pepineiro. Pesquisa Agropecuária Brasileira 46: 1609–1618 [Google Scholar]
- Silva-Mann R, Carvalho Vieira MGG, Machado JC, Bernandino Filho JR, Salgado KCC, Stevens MR. (2005). AFLP markers differentiate isolates of Colletotrichum gossypii from C. gossypii var. cephalosporioides. Fitopatologia Brasileira 30: 169–172 [Google Scholar]
- Simmonds JH. (1965). A study of the species of Colletotrichum causing ripe fruit rots in Queensland. Queensland Journal of Agricultural and Animal Sciences 22: 437–459 [Google Scholar]
- Simmonds JH. (1968). Type specimens of Colletotrichum gloeosporioides var. minor and Colletotrichum acutatum. Queensland Journal of Agricultural and Animal Sciences 25: 178A [Google Scholar]
- Singh UP. (1974). A new forma specialis of Colletotrichum gloeosporioides Penz. from India. Beihefte zur Nova Hedwigia 47: 451–452 [Google Scholar]
- Singh UP. (1975). Colletotrichum gloeosporioides Penzig - A mycoparasite on Ravenelia sessilis Berkeley. Current Science 44: 623–624 [Google Scholar]
- Singh RD, Prasad N, Mathur RL. (1966). On the taxonomy of the fungus causing anthracnose of Dioscorea alata L. Indian Phytopathology 19: 65–71 [Google Scholar]
- Small W. (1926). On the occurrences of a species of Colletotrichum. Transactions of the British Mycological Society 11: 112–137 [Google Scholar]
- Snowdon AL. 1991. A colour atlas of post-harvest diseases and disorders of fruit and vegetables. Volume 2. Vegetables. Wolfe Scientific; [Google Scholar]
- Sosa de Castro NT, Cabrera de Alvarez MG, Alvarez RE. (2001). Primera información de Colletotrichum camelliae como patógeno de Camellia japonica, en Corrientes. Retrieved 6 Oct 2010 from www1.unne.edu.ar/cyt/2001/5-Agrarias/A-056.pdf
- Southworth EA. (1891). Antracnose of cotton. Journal of Mycology 6: 100–105 [Google Scholar]
- Spaulding P. (1958). Diseases of foreign trees growing in the United States, an annotated list. USDA Agriculture Handbook 139. [Google Scholar]
- Spegazzini CL. (1878). Ampelomiceti Italici, ossia enumerazione, diagnosi e storia dei principali parassiti della vite. Rivista Viticoltura ed Enologia 2: 405–411 [Google Scholar]
- Sreenivasaprasad S, Mills PR, Meehan B M, Brown AE. (1996). Phylogeny and systematics of 18 Colletotrichum species based on ribosomal DNA spacer sequences. Genome 39: 499–512 [DOI] [PubMed] [Google Scholar]
- Stephenson SA, Green JR, Manners JM, Maclean DJ. (1997). Cloning and characterisation of glutamine synthetase from Colletotrichum gloeosporioides and demonstration of elevated expression during pathogenesis on Stylosanthes guianensis. Current Genetics 31: 447–454 [DOI] [PubMed] [Google Scholar]
- Stevens FL, Hall JG. (1909). Eine neue Feigen-Anthraknose (Colletotrichose). Zeitschrift für Pflanzenkrankheiten 19: 65–68 [Google Scholar]
- Stoneman B. (1898). A comparative study of the development of some anthracnoses. Botanical Gazette 26: 69–120 [Google Scholar]
- Su YY, Noireung P, Liu F, Hyde KD, Moslem MA, et al. (2011). Epitypification of Colletotrichum musae, the causative agent of banana anthracnose. Mycoscience 52: 376–382 [Google Scholar]
- Sutton BC, Waterston JM. (1970). Colletotrichum musae. CMI Descriptions of Plant Pathogenic Fungi and Bacteria No. 222 [Google Scholar]
- Sutton BC. (1980). The Coelomycetes. Commonwealth Mycological Institite; Kew: [Google Scholar]
- Sutton BC. (1992). The genus Glomerella and its anamorph Colletotrichum. In: Colletotrichum Biology, Pathology and Control. (Bailey JA, Jeger MJ, eds). CAB International, Wallingford: 1–26 [Google Scholar]
- Talhinas P, Sreenivasaprasad S, Neves-Martins J, Oliviera H. (2005). Molecular and phenotypic analyses reveal association of diverse Colletotrichum acutatum groups and a low level of C. gloeosporioides with olive anthracnose. Applied and Environmental Microbiology 71: 2987–2998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor JW, Jacobson DJ, Kroken S, Kasuga T, Geiser DM, et al. (2000). Phylogenetic species recognition and species concepts in fungi. Fungal Genetics and Biology 31: 21–32 [DOI] [PubMed] [Google Scholar]
- Taylor JW, Berbee ML. (2006). Dating divergences in the Fungal Tree of Life: review and new analyses. Mycologia 98: 838–849 [DOI] [PubMed] [Google Scholar]
- TeBeest DO. (1988). Additions to host range of Colletotrichum gloeosporioides f. sp. aeschynomene. Plant Disease 72: 16–18 [Google Scholar]
- Templeton MD, Rikkerink EHA, Solon SL, Crowhurst RN. (1992). Cloning and molecular characterization of the glyceraldehyde-3-phosphate dehydrogenase-encoding gene and cDNA from the plant pathogenic fungus Glomerella cingulata. Gene 122: 225–230 [DOI] [PubMed] [Google Scholar]
- Than PP, Jeewon R, Hyde KD, Pongsupasamit S, Mongkolporn O, Taylor PWJ. (2008). Characterisation and pathogenicity of Colletotrichum species asscoaited with anthracnose on chilli (Capsicum spp.) in Thailand. Plant Pathology 57: 562–572 [Google Scholar]
- Thang MM. (2008). Biodiversity survey of coelomycetes in Burma. Australasian Mycologist 27: 74–110 [Google Scholar]
- Trujillo EE, Latterell FM, Rossi AE. (1986). Colletotrichum gloeosporioides, a possible biological control agent for Clidemia hirta in Hawaiian forests. Plant Disease 70: 974–976 [Google Scholar]
- Tsurushima T, Ueno T, Fukami H, Irie H, Inoue M. (1995). Germination of self-inhibitors from Colletotrichum gloeosporioides f. sp. jussiaea. Molecular Plant-Microbe Interactions 8: 652–657 [Google Scholar]
- Vajna L, Bagyinka T. (2002). Occurrence of privet anthracnose in Hungary caused by Glomerella cingulata. Plant Disease 51: 403 [Google Scholar]
- Varzea VMP, Rodrigues Junior CJ, Lewis BG. (2002). Distinguishing characteristics and vegetative compatibility of Colletotrichum kahawae in comparison with other related species from coffee. Plant Pathology 51: 202–207 [Google Scholar]
- Venkatakrishniah NS. (1952). Glomerella psidii (Del.) Sheld. and Pestalotia psidii Pat. associated with a cankerous disease of guava. Proceedings of the Indian Academy of Plant Sciences 36: 129–134 [Google Scholar]
- Vermeulen H, De Ferrante B, Gielink AJ. (1984). Genetic and morphological diversity of mono-spore isolates of Glomerella cingulata associated with coffee berry disease. Netherlands Journal of Plant Pathology 90: 213–223 [Google Scholar]
- Vinijsanun T, Irwin JAG, Cameron DF. (1987). Host range of three strains of Colletotrichum gloeosporioides from tropical pasture legumes, and comparative histological studies of interactions between Type B disease-producing strains and Stylosanthes scabra (non-host) and S. guianensis (host). Australian Journal of Botany 35: 665–677 [Google Scholar]
- Waller JM, Bridge PD, Black R, Hakiza G. (1993). Characterisation of the coffee berry disease pathogen, Colletotrichum kahawae sp. nov. Mycological Research 97: 989–994 [Google Scholar]
- Wang XX, Wang B, Liu JL, Chen J, Cui XP, et al. (2010). First reports of anthracnose caused by Colletotrichum gloesporioides on ramie in China. Plant Disease 94: 1508 [DOI] [PubMed] [Google Scholar]
- Weir BS, Johnston PR. (2009). Defining and delimiting genetic species in Colletotrichum. Asian Mycologyical Congress 2009. Taichung Taiwan November 15–19 pg O-128 [Google Scholar]
- Weir BS, Johnston PR. (2010). Characterisation and neotypification of Gloeosporium kaki Hori as Colletotrichum horii nom. nov. Mycotaxon 111: 209–219 [Google Scholar]
- Weir BS, Johnston PR. (2011). Towards a secondary fungal barcode for Colletotrichum [Sordariomycetes, Ascomycota]. Forth international barcode of life conference, Adelaide Australia, 28 November – 3 December 2011 [Google Scholar]
- White TJ, Bruns T, Lee S, Taylor JW. (1990). Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: PCR Protocols: A Guide to Methods and Applications. (Innis MA, Gelfand DH, Sninsky JJ, White TJ. eds). Academic Press: New York: 315–322 [Google Scholar]
- Wickham H. (2009). ggplot2: elegant graphics for data analysis. Springer; New York: [Google Scholar]
- Wikee S, Cai L, Pairin N, McKenzie EHC, Su YY, et al. (2011). Colletotrichum species from Jasmine (Jasminum sambac). Fungal Diversity 46: 171–182 [Google Scholar]
- Willis JC. (1899). DCLII - Tea and coffee diseases. Bulletin of Miscellaneous Information Royal Botanical Gardens Kew 1899: 89–94 [Google Scholar]
- Winch JE, Newhook FJ, Jackson GVH, Cole JS. (1984). Studies of Colletotrichum gloeosporioides disease on yam, Dioscorea alata, in Solomon Islands. Plant Pathology 33: 467–477 [Google Scholar]
- Wollenweber HW, Hochapfel H. (1949). Beiträge zur Kenntnis parasitärer und saprophytischer Pilze. VI. Vermicularia, Colletotrichum, Gloeosporium, Glomerella und ihre Beziehung zur Fruchtfäule. Zeitschrift für Parasitenkunde 14: 181–268 [Google Scholar]
- Xie L, Zhang JZ, Cai L, Hyde KD. (2010a). Biology of Colletotrichum horii, the causal agent of persimmon anthracnose. Mycology 1: 242–253 [Google Scholar]
- Xie L, Zhang JZ, Wan Y, Hu DW. (2010b). Identification of Colletotrichum spp. isolated from strawberry in Zhejiang Province and Shanghai City, China. Journal of Zhejiang University Science B (Biomedicine & Biotechnology) 11: 61–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto W. (1960). A revision of the genus and species names of anthracnose fungi in Japan. Plant Protection 14: 49–52 [in Japanese] [Google Scholar]
- Yan J, Wu PS, Du HZ, Zhang QE. (2011). First report of black spot caused by Colletotrichum gloeosporioides on paper mulberry in China. Plant Disease 95: 880 [DOI] [PubMed] [Google Scholar]
- Yang SY, Su SC, Liu T, Fan G, Wang J. (2011). First report of anthracnose caused by Colletotrichum gloeosporioides on pistachio (Pistacia vera) in China. Plant Disease 95: 1314 [DOI] [PubMed] [Google Scholar]
- Yang YL, Liu ZY, Cai L, Hyde KD, Yu ZN, McKenzie EHC. (2009). Colletotrichum anthracnose of Amaryllidaceae. Fungal Diversity 39: 123–146 [Google Scholar]
- Zhang TY. (1985). A forma specialis of Colletotrichum gloeosporioides on Cuscuta sp. Acta Mycologica Sinica 4: 223–239 [In Chinese] [Google Scholar]
- Zhang X, Yang G, Yang J, Shu Q. (2012). Physiological mechanism of resistance to anthracnose of different Camellia varieties. African Journal of Biotechnology 11: 2026–2031 [Google Scholar]