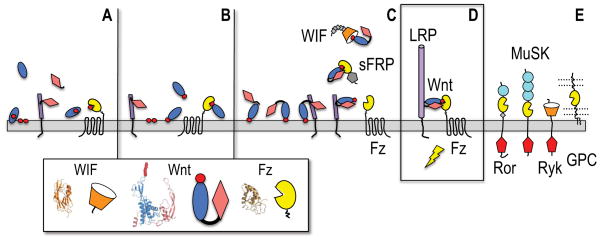
Figure 3. Evolutionary model for the emergence of Wnts as Fz receptor-binding morphogens.
(A) A membrane-interacting saposin-like domain (blue oval) has a (red circle) lipid-loading role for a Fz-like GPCR. A secreted cystine knot cytokine (pink diamond) binds a cell surface LRP-like progenitor (purple cylinder).
(B) The saposin-like domain gains a Ser-acylation site in its membrane-interacting loops and can then directly bind the Fz domain of the GPCR.
(C) A hydrophobic motif in the cytokine N-terminal extension can dock to the saposin helical domain (Figure S1), and a fortuitous gene fusion of these interacting proteins creates a two domain Wnt progenitor that retains cytokine-mediated binding to a primitive LRP, and can be presented together to the Fz GPCR principally by lipid docking. They cytokine portion of the Wnt has an evolved affinity for the interacting Fz and WIF domains, on the receptors or soluble binding proteins (sFRPs, WIF). Small grey diamonds mark the EGF repeat tail in WIF, while a grey pentagon in sFRP illustrates a C-terminal netrin module.
(D) The Wnt remains the critical bridging element in the active ternary receptor complex with LRP5/6 and a Fz GPCR. The purple cylinder in the LRP chain (or its shorter progenitor LRP) denotes the four-fold-repeat of β-propeller and EGF domains.
(E) Additional signaling receptors (Ror, MuSK and Ryk) and presentation molecules (glypican or GPC) can potentially engage lipidated Wnts. Light blue circles in Ror and MuSK ectodomains mark Ig modules, while intracellular tyrosine kinases are noted by red pentagons.