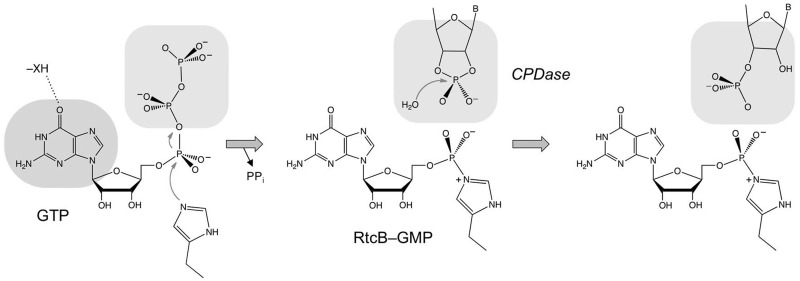
Figure 9.
Scheme for the GTP-dependent 2′,3′-CPDase activity of RtcB. The model posits that guanylylation of RtcB on His337 precedes the CPDase reaction. The histidine-GMP adduct is depicted with a P–Nε covalent bond, although it is not yet established whether the nucleophilic atom is histidine Nε or Nδ. The nucleobase specificity for a 6-oxopurine is likely dictated by hydrogen-bond donation from an enzymic moiety—XH. The model assumes that the binding pocket for the GTP β and γ phosphates (the PPi leaving group in the RtcB guanylylation reaction) overlaps that for the RNA 2,′3′-cyclic phosphate terminus in the CPDase reaction.