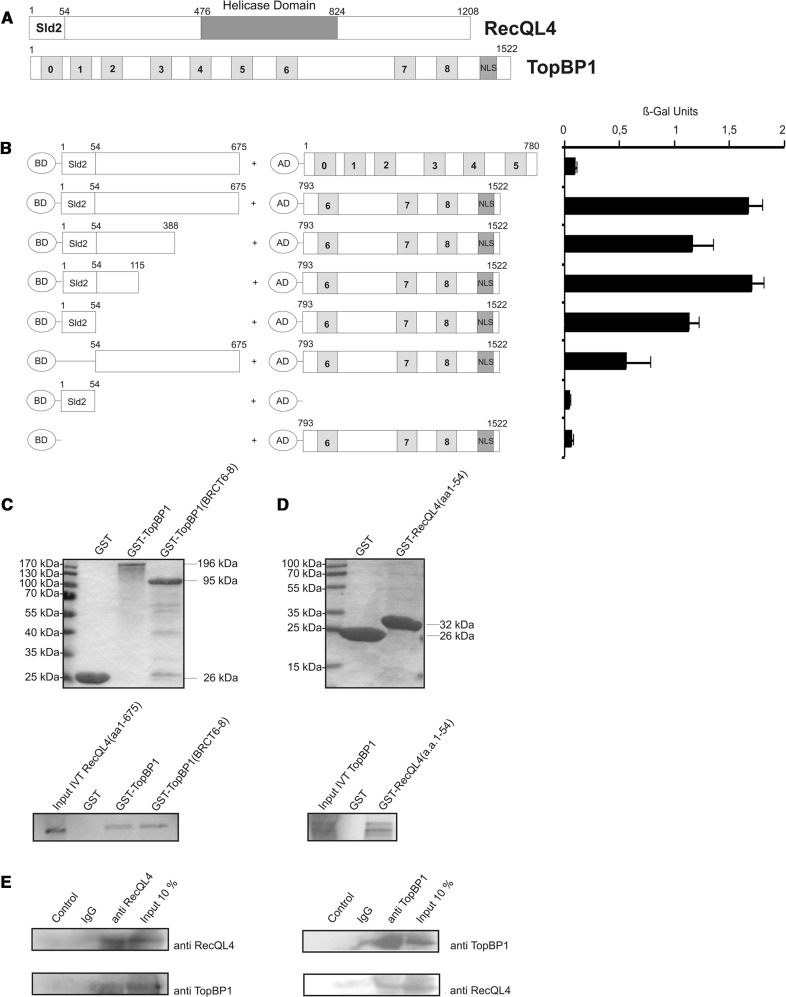
Figure 1.
The Sld2-homologous N-terminal domain of RecQL4 interacts directly with TopBP1. (A) Schematic depiction of human RecQL4 and TopBP1. (B) Specific interaction of the N-terminal domain of RecQL4 fused to the GAL4 DNA-BD with C-terminal part (a.a. 793–1522) of TopBP1 fused to the GAL4-AD in yeast. Interaction was semi-quantitatively assessed by β-galactosidase liquid culture activity assays. β-galactosidase values represent the mean and standard deviations of three independent experiments. (C) GST pull-down experiments performed with purified GST, GST–TopBP1 and GST–TopBP1 (a.a. 793–1522) and [35S]–methionine–labelled RecQL4 (a.a. 1–675) (lower panel). Coomassie brilliant blue R250-stained SDS–PAGE gels of the purified GST constructs are presented (upper panel). (D) GST pull-down experiments performed with purified GST and GST–RecQL4 (a.a. 1–54) and [35S]-cysteine-labelled full-length TopBP1. Coomassie brilliant blue R250-stained SDS–PAGE gels of the purified GST constructs are presented (upper panel). Input IVT: 10 µl of the in vitro translation product. (E) Reciprocal immunoprecipitation of RecQL4 and TopBP1. Cell extracts from human Hek293 cells transiently co-transfected with expression vectors for RecQL4 and ToPBP1 were subjected to immunoprecipitation using antibodies against RecQL4 and TopBP1 as described in ‘Material and Methods’. The precipitates were then analysed by SDS–PAGE and Western blot against the cognate proteins. Controls without antibody or with non-specific rabbit IgG served as negative control and 10% of the input served as an indicator for the (co-)immunoprecipitation efficiency.