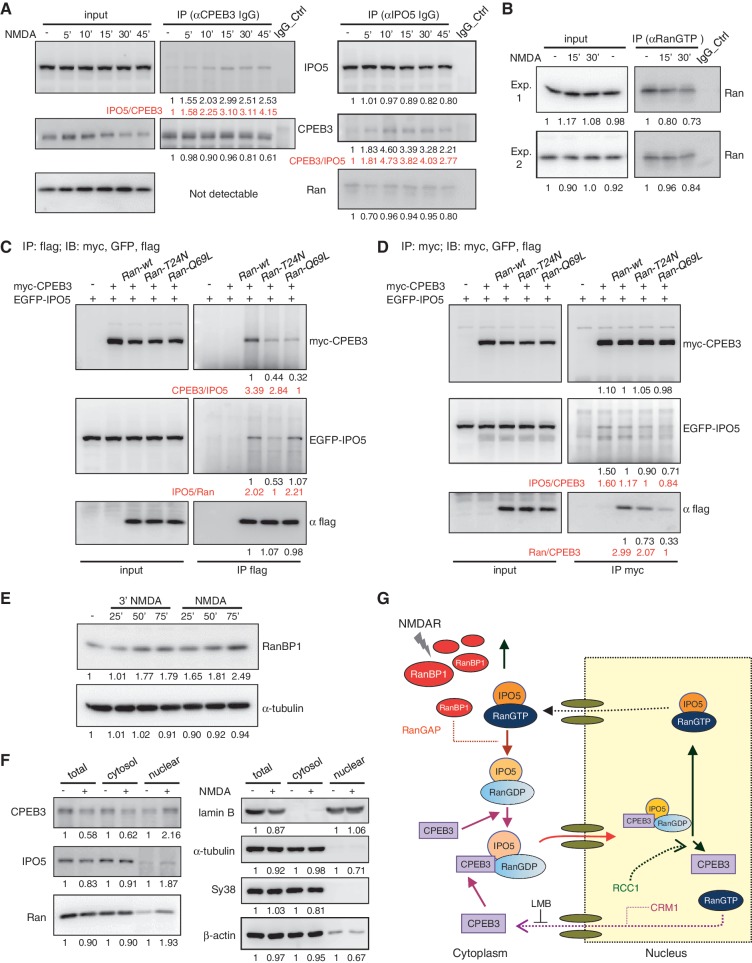
Figure 7.
NMDAR signaling enhances CPEB3–IPO5 interaction through Ran-related changes. (A) Neurons stimulated with 3 min of NMDA and then incubated for the denoted time were used for IP with CPEB3 and IPO5 IgGs, followed by immunodetection with IPO5, CPEB3 and Ran antibodies. The intensities of immunodetected signals analysed by the ImageJ software are displayed as relative ratios at the bottoms of blots. The ratios of the co-precipitated target versus the immunoprecipitated protein (IPO5/CPEB3 or CPEB3/IPO5) are shown in red. (B) The cytoplasmic fraction isolated from neurons treated with ± NMDA was precipitated with the RanGTP antibody and immunoblotted with Ran antibody. (C and D) HeLa cells expressing myc-CPEB3, EGFP-IPO5 along with Ran-wt, T24N or Q69L mutant were pulled down with (C) flag or (D) myc antibodies. The precipitated substances were probed with GFP, myc and flag antibodies. (E) Neurons stimulated with a pulse of NMDA or NMDA for the indicated times were harvested for western blotting using RanBP1 and tubulin antibodies. (F) The nuclear and cytosol lysates fractionated from neurons treated with ± NMDA for 30 min were used for immunoblotting. (G) Schematic model of NMDA-induced nuclear import of CPEB3. NMDAR signaling increases RanBP1 expression, combined with RanGAP to stimulate Ran’s GTPase activity. Consequently, the IPO5-RanGDP complex can bind to CPEB3 and deliver CPEB3 to the nucleus. After nuclear import, a high RanGTP concentration and RCC1 in the nucleus triggers the release of CPEB3 from the ternary complex because RanGTP impairs the interaction between IPO5 and CPEB3. The nuclear export of CPEB3 is mediated by CRM1.