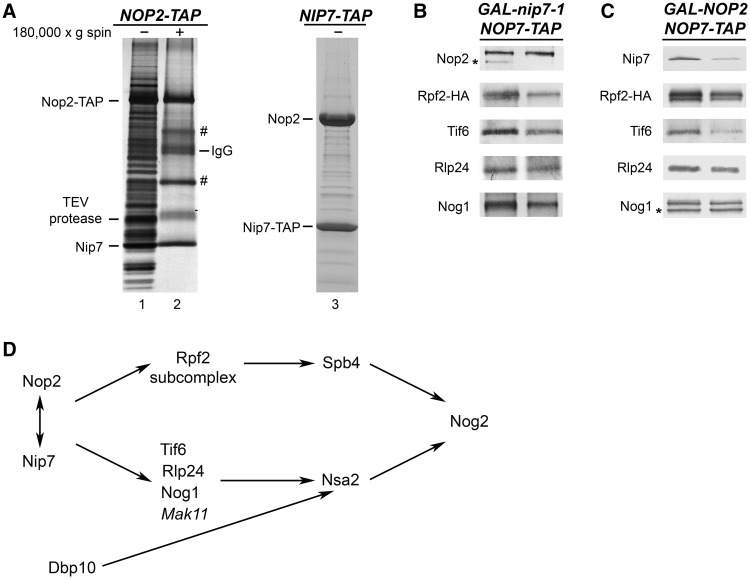
Figure 5.
Nop2 and Nip7 form a subcomplex, are interdependent for assembly into pre-ribosomes and function in recruiting of other B-factors. (A) Nop2 and Nip7 form a heteromer in vivo. Yeast strains expressing TAP-tagged Nop2 or Nip7 were grown in YEPGlu medium to 3 × 107 cells/ml. Whole-cell extracts from the NOP2-TAP strain (lane 1) or the supernatants after high-speed centrifugation (lane 2) were subjected to affinity purification using Nop2-TAP, to purify Nop2-containing pre-ribosomes or subcomplexes, respectively. Purified proteins were identified by mass spectrometry. Proteins labeled with ‘#’ are common contaminants in purified samples. Whole-cell extract from the NIP7-TAP strain was subjected to affinity purification using Nip7-TAP (lane 3). Nop2 and Nip7 in these purified samples were identified by mass spectrometry. (B and C) Nop2 and Nip7 are interdependent for their assembly into pre-rRNPs and important for incorporation of other B-factors into pre-ribosomes. GAL-nip7-1 or GAL-NOP2 yeast were grown in YEPGal or shifted to YEPGlu. Whole-cell extracts were subjected to affinity purification, using Nop7-TAP to isolate pre-ribosomes. Amounts of B-factors present in the purified pre-ribosomes were analyzed by western blotting. Each pair of samples is shown as described in Figure 3A. Western blots showing the depletion of Nop2 or Nip7 from preribosomes and the Nop7-TAP loading control are shown in Figure 3A. (D) Nop2 and Nip7 are important for the stable association of all the B-factors.