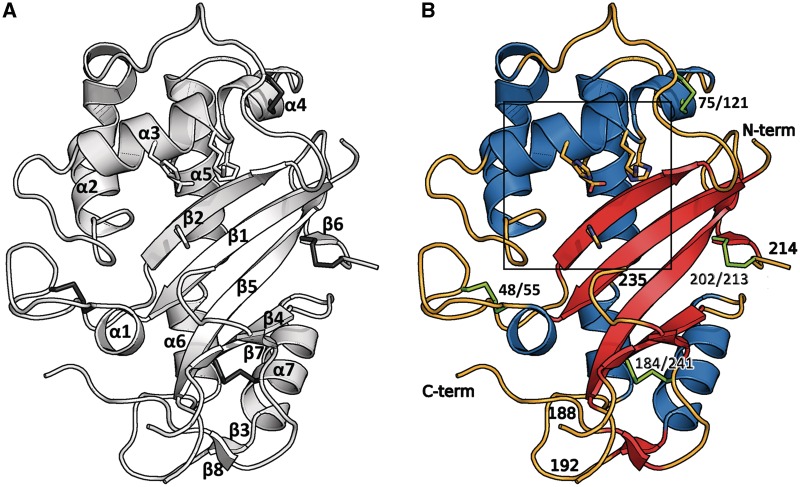
Figure 2.
The structure shows a typical α+β core fold. The catalytic residues are shown as sticks. (A) Topology of human RNase T2: the structure shows the typical fold of this family with seven α-helices and eight β-strands compromising two anti-parallel sheets. (B) Residues 188–192 and 214–235 and the terminal ends are disordered and could not be resolved in the electron density. Disulfide bridges are shown in green. The box refers to Figure 6.