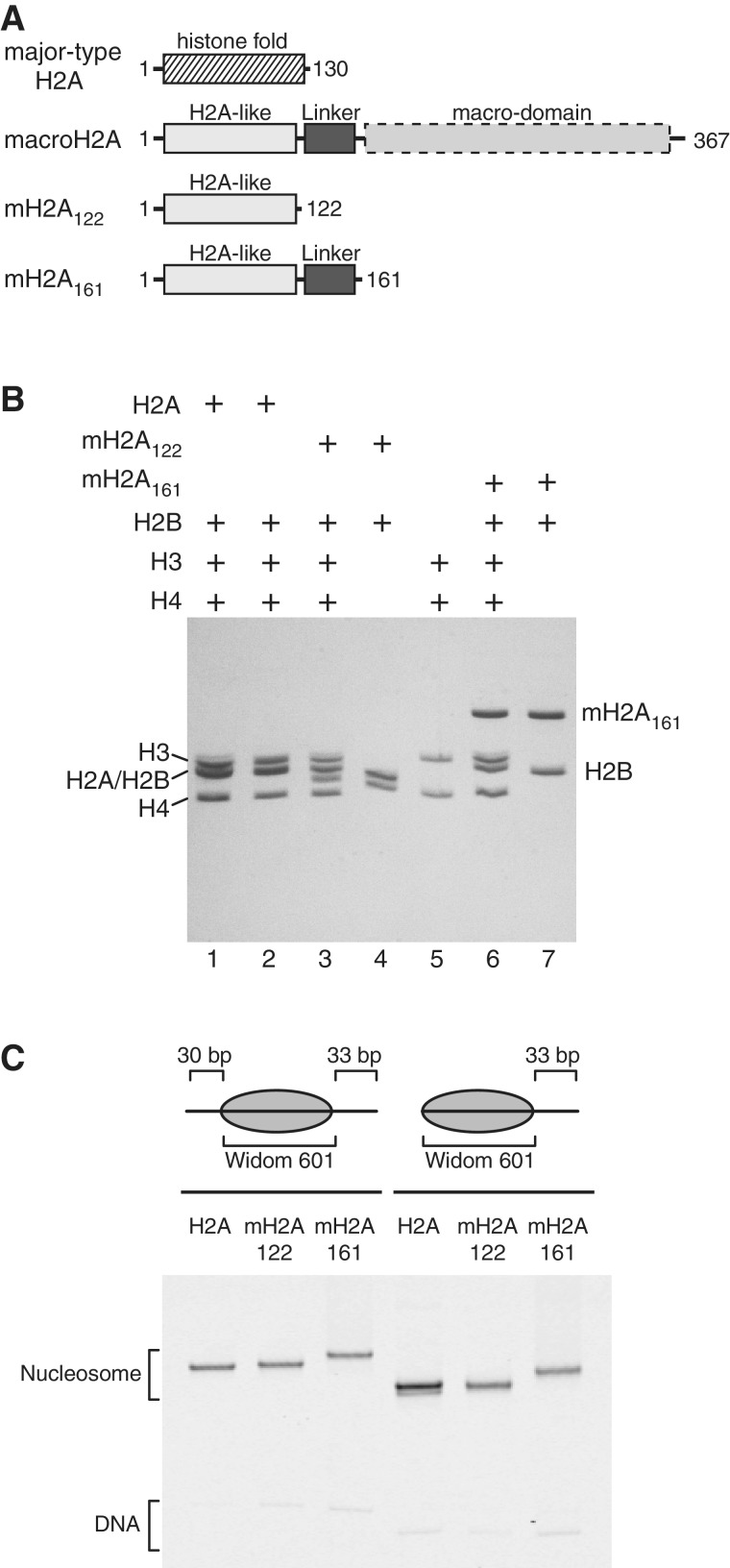
Figure 1.
Overview of nucleosomes used in this study. (A) Domain organization of full-length macroH2A, and the truncations used in this study compared with major-type H2A. The truncated macroH2A construct, mH2A122, possesses only the histone-fold portion of macroH2A, and mH2A161 additionally contains the basic macro-linker. (B) Nucleosomes and histone oligomers analyzed by SDS PAGE. To confirm the sizes and ratios of histone proteins in reconstituted nucleosomes, nucleosomes containing major-type H2A (lane 1), mH2A122 (lane 3) and mH2A161 (lane 6) were run alongside the histone components: the histone octamer with major-type H2A (lane 2), mH2A122-H2B dimer (lane 4), (H3-H4)2 tetramer (lane 5) and mH2A161-H2B dimer (lane 7). (C) Nucleosomes analyzed by native PAGE. Fluorescently labeled nucleosomes (centered 30-N-33 and end-positioned 0-N-33) were produced with either major-type H2A, mH2A122 or mH2A161 as indicated.