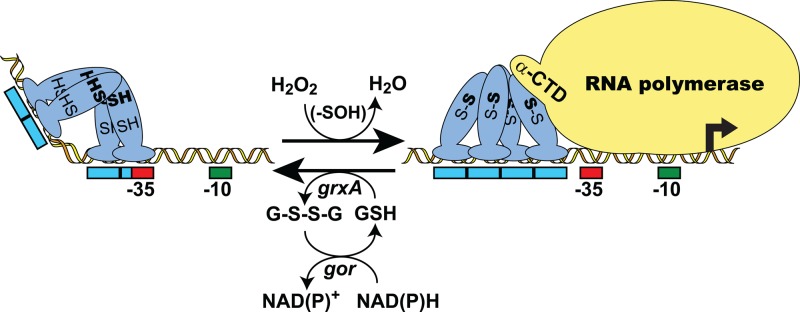
Fig 1.
Mechanism of transcription activation via intramolecular disulfide formation in 2-Cys OxyR. A model for H2O2-dependent, OxyR-mediated transcription activation of a target gene in E. coli is shown. Activation begins with the oxidation of the sensing cysteine (SH) residue of OxyR to sulfenic acid (-SOH), followed by the rapid formation of an intramolecular disulfide bond with the resolving cysteine (SH). The resulting conformational change often causes a shift in the DNase I footprint and can also affect DNA binding affinity and promoter conformation as well as render OxyR capable of interacting with RNA polymerase. Transcription activation involves the direct interaction of OxyR with the alpha subunit C-terminal domain (α-CTD) of RNA polymerase. Oxidized OxyR is reduced, using reduced glutathione (GSH) as the electron donor, via the glutaredoxin (grxA)/glutathione reductase (gor) system, with reducing equivalents ultimately supplied by NAD(P)H. Red and green boxes indicate RNA polymerase σ70 −35 and −10 promoter elements, respectively. Blue boxes indicate OxyR DNA binding contacts. Activation can also occur via oxidative modification of the sensing cysteine alone.