Table 2.
Library of tetrahydro-β-carbolinecyclobutenes 2{1,3}–2{1,8}.
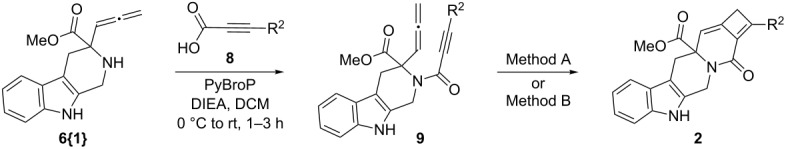 | ||||||
| entry | R2 | yield of 9a (%) | methodb | yield of 2a (%) | purityc | |
| 1 | 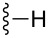 |
8{1} | 0 9{1,1} | — | — | — |
| 2 | 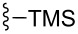 |
8{2} | 64 9{1,2} | A | NDd | — |
| 3 | 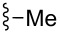 |
8{3} | 75e 9{1,3} | A | 73 2{1,3} | 99% |
| 4 | 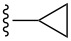 |
8{4} | 71 9{1,4} | A | 57 2{1,4} | 99% |
| 5 | 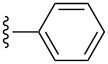 |
8{5} |
9{1,5} 2{1,5} |
A B |
57f
2{1,5} 40 2{1,5} |
99% |
| 6 | 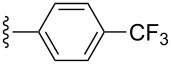 |
8{6} |
9{1,6} 2{1,6} |
B | 41 2{1,6} | 99% |
| 7 | 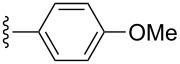 |
8{7} |
9{1,7} 2{1,7} |
B | 30 2{1,7} | 99% |
| 8 | 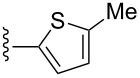 |
8{8} | 9{1,8} | B | 33 2{1,8} | 99% |
aIsolated yield; bmethod A: μW, 160 °C, DMF, 10 min; method B: Placed in front of two 6 W UV lamps (245 nm), CH2Cl2, rt, 16 h, no stirring; cpurity established by LCMS/ELS; dND = not detected; eμW, 225 °C, DMF, 7 min, (39%); fseparated 9{1,5} (57% yield) from 2{1,5} (18% yield) after the coupling reaction then submitted 9{1,5} to method A to give 2{1,5} in 68% yield. This compound was recombined with the previously isolated 2{1,5} to afford a combined 57% yield.
