Table 4.
Potential targets of β-carbolines based upon bioactivity data in ChEMBL.
| target | CHEMBL compound | bioactivity type and reference |
our compound | similarity score |
| C–C chemokine receptor type 3 |
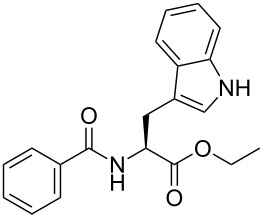 CHEMBL33838 |
IC50 = 325 nM [21] |
 A |
0.79 |
| Leishmania donovani |
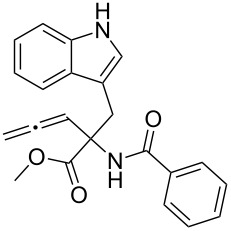
 CHEMBL55830 |
IC50 = 1.42 µM [22] |
 4 |
0.80 |
| Acanthocheilonema viteae |
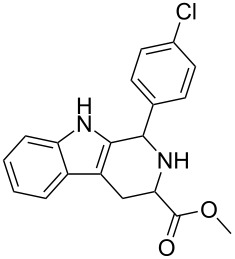 CHEMBL44573 |
Activity = 94% [23] |
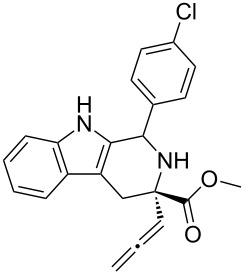 6{5} |
0.86 |
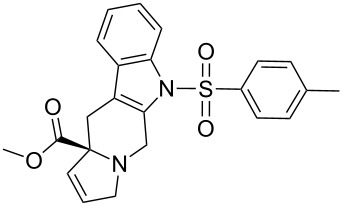
| Gamma-aminobutyric acid receptor subunit gamma-2 |
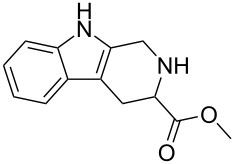 CHEMBL358326 |
IC50 = 250 nM [24] |
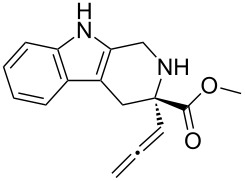 6{1} |
0.84 |
| 5-Hydroxytryptamine receptor 6 |
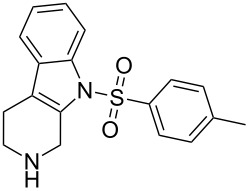 CHEMBL370935 |
Ki = 271.3 nM [25] |
 7{1} |
0.69 |
| 3-Hydroxyacyl-CoA dehydrogenase type-2 |
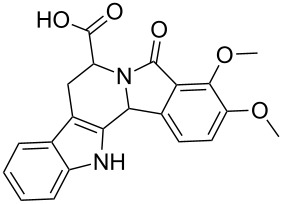 CHEMBL1382101 |
Potency = 31.6 µM PubChem AID:893 |
 7{7} |
0.64 |
| Benzodiazepine receptors |
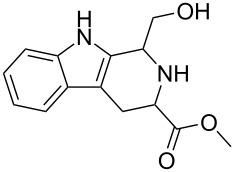 CHEMBL11901 |
Ki = 510 nM [26] |
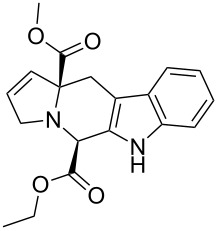 1{16} |
0.72 |
| Angiotensin-converting enzyme |
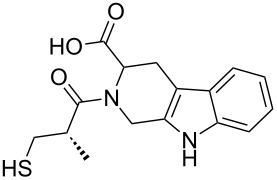 CHEMBL148616 |
IC50 = 500 nM [27] |
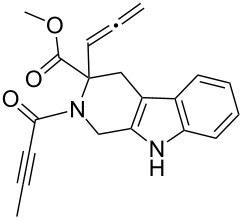 9{1,3} |
0.71 |
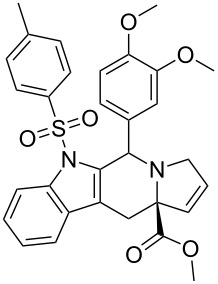
| Antithrombotic potency |
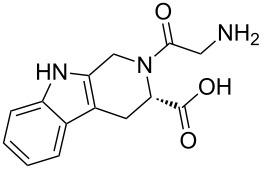 CHEMBL1089460 |
IC50 = 8.56 nM [28] |
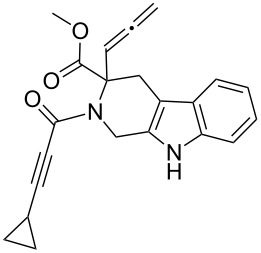 9{1,4} |
0.69 |
| Mitogen-activated protein kinase 1 |
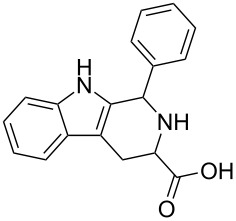 CHEMBL44295 |
Potency = 0.794 µM PubChem AID:995 |
 6{4} |
0.82 |
| Breast adenocarcinoma cells |
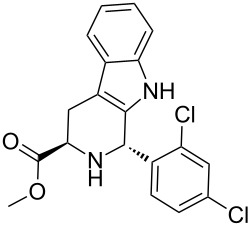 CHEMBL1650665 |
Inhibition = 78% [29] |
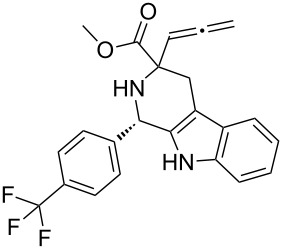 6{6} |
0.79 |
| DNA polymerase iota |
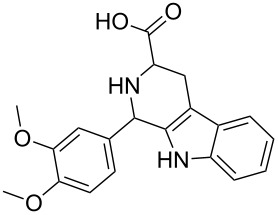 CHEMBL1360719 |
Potency = 1.78 µM PubChem AID:588590 |
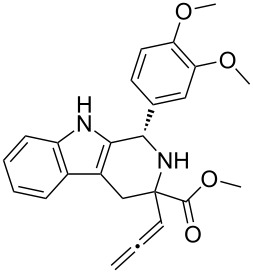 6{7} |
0.85 |
