Table 1.
Optimization studies for the SNAr reaction utilizing sultam 4.
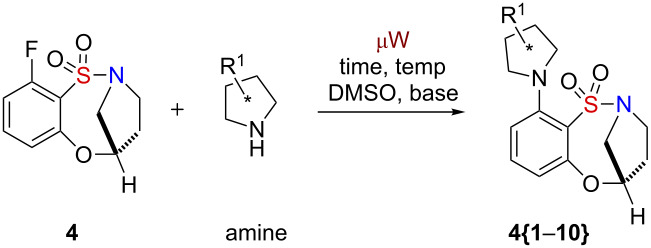 | |||||||
| Entry | Amine | Equiv | Base | Conc. (M) | Time (min) | Temp (°C) | Yielda (%) |
| 1 | (R)-3-pyrrolidinol | 1.3 | Cs2CO3 | 0.1 | 30 | 150 | NA |
| 2 | (R)-3-pyrrolidinol | 4.4 | — | 0.1 | 20 | 150 | 94 |
| 3 | (S)-2-pyrrolidine methanol | 4.4 | — | 0.1 | 30 | 150 | 29 |
| 4 | (S)-2-methoxymethyl pyrrolidine | 4.3 | — | 0.1 | 50 | 180 | NA |
| 5 | (S)-3-dimethylamino pyrrolidine | 5.0 | — | 0.1 | 50 | 180 | 88 |
| 6 | (R)-2-methylpyrrolidine | 5.0 | — | 0.1 | 30 | 150 | 42 |
| 7 | (R)-2-methylpyrrolidine | 5.0 | — | 0.1 | 40 | 180 | 62b |
| 8 | (R)-2-methylpyrrolidine | 5.0 | — | 0.1 | 50 | 180 | 70 |
| 9 | (R)-2-methylpyrrolidine | 5.0 | — | 0.1 | 60 | 180 | 35 |
| 10 | (R)-2-methylpyrrolidine | 5.0 | — | 0.5 | 50 | 180 | 95b |
| 11 | (R)-2-methylpyrrolidine | 5.0 | — | 1.0 | 50 | 180 | 83b |
aYields are reported after flash column chromatography on silica gel. bCrude yield as judged by 1H NMR.
