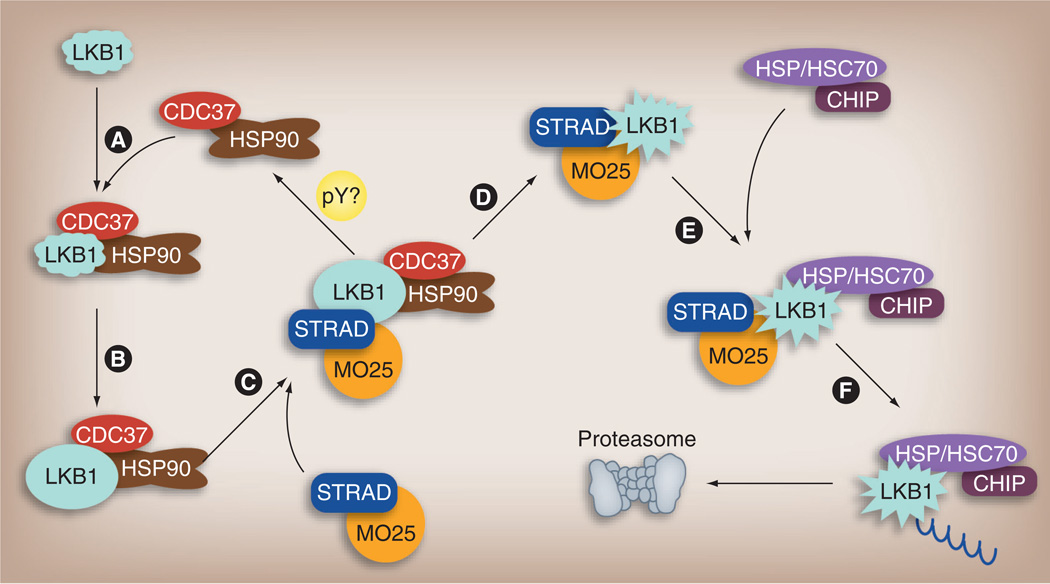
Figure 1. Activity and protein stability of the LKB1 kinase are regulated by molecular chaperones.
The HSP90–CDC37 chaperone complex binds to nascent LKB1 protein (A) and assists its correct folding (B). Folded LKB1 remains bound to this chaperone complex in a stable but inactive state. Association with the STRAD–MO25 heterodimer leads to LKB1 activation (C), but also to dissociation from the HSP90–CDC37 complex (possibly promoted by tyrosine phosphorylation) (D). LKB1 that is not in complex with HSP90–CDC37 is available to bind to the chaperone HSP/HSC70, which recruits the ubiquitin ligase CHIP (E). CHIP ubiquitinates LKB1 (F), leading to its proteasome-mediated degradation. Thus, LKB1 activation and degradation are finely balanced events regulated by dynamic chaperone interactions.