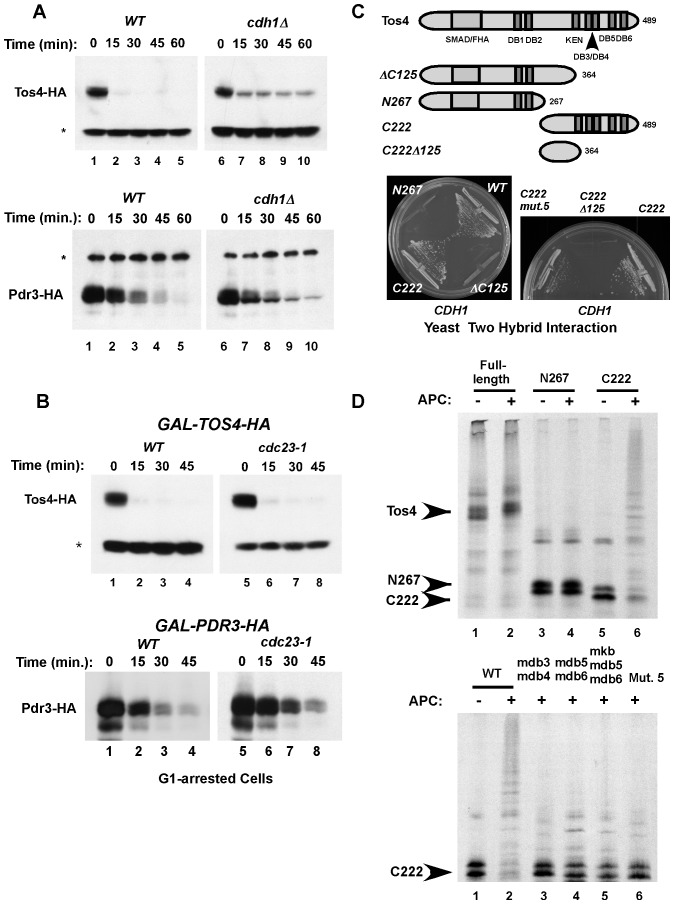
Figure 5. Tos4 and Pdr3 are potential APC/CCdh1 substrates.
(A) Tos4 and Pdr3 are partially stabilized in cdh1Δ cells. Asynchronously growing WT or cdh1Δ cells expressing Tos4-HA or Pdr3-HA were treated with cycloheximide for the indicated times. HA-fusion protein levels from the cell extracts were determined by immunoblotting with anti-HA antibodies. A non-specific cross-reactive protein (*) served as a loading control. (B) Overexpressed Pdr3-HA but not Tos4-HA was partially stabilized in an APC/C mutant strain. Cells were arrested in G1 and induced to express the indicated HA-tagged protein by galactose addition. Cells were shifted to 37°C to inactivate the APC/C in the cdc23-1 mutant cells, and then treated with cycloheximide for the indicated times prior to extract preparation and protein detection by immunoblotting with anti-HA antibodies. (C) The carboxyl-terminal region (C222) of Tos4 contains multiple motifs that mediate interaction with Cdh1 in the yeast two-hybrid system. Top: Schematic of Tos4 constructs showing the locations of the putative KEN box and DB motifs. Tos4-mut. 5 contains mutations of the four C-terminal D-box and one KEN box motifs. (D) Tos4-C222 was efficiently ubiquitinated by APC/CCdh1 in vitro. The N267 and C222 forms of Tos4 are shown in (C), as are the sites of the various DB mutations. Locations of the putative Tos4 degrons are as follows: DB1, 232; DB2, 237; KEN, 365; DB3, 414; DB4, 418; DB5, 458; DB6, 469.