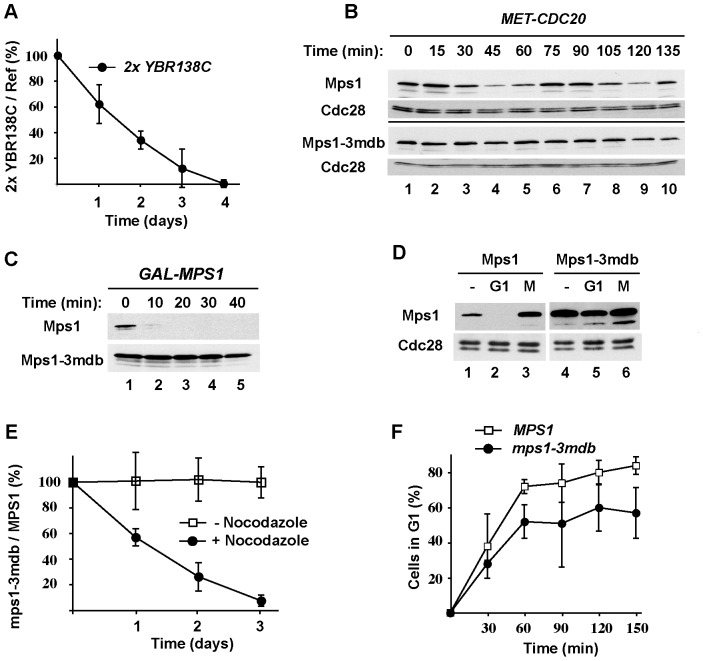
Figure 6. Stabilization of Ybr138C or Mps1 reduces cell fitness.
(A) Co-cultures of wild-type and 2xYBR138C-TAP cells. Cells carrying two copies of the YBR138C gene were genetically marked (with TRP1) and grown together with wild-type (W303) cells (marked with LEU2) in complete medium for four days with dilution of the co-cultures once per day. The cultures were tested daily for the presence of TRP1 and LEU2 auxotrophs by plating on selective media. The markers were swapped, the experiment repeated, and the averaged results of two trials were plotted. The fraction of 2xYBR138C-TAP cells on day 0 was arbitrarily set to 100%. (B) Cell cycle degradation of Mps1 and Mps1-3mdb. MET-CDC20 cells carrying endogenously expressed TAP-tagged MPS1 or mps1-3mdb were synchronized in mitosis by incubation with 5 mM methionine for 2.5 hours to deplete Cdc20. Cells were released from the arrest into methionine-free medium. Samples were taken at the indicated times after release and processed for blotting to detect TAP-tagged proteins. (C) Stabilization of Mps1 by mutation of its D-boxes. The stabilities of Mps1 and Mps1-3mdb in G1 were assessed as in Figure 2A. (D) Elevated expression of Mps1-3mdb. Cells carrying endogenously expressed Mps1 or Mps1-3mdb were arrested in G1 and M phase. The levels of Mps1-TAP were visualized by immunoblotting. Lanes 1 and 4 show the levels of these proteins in cell extracts prepared from asynchronous cell cultures. (E) Expression of Mps1-3mdb reduces cell fitness in the presence of mild spindle disruption. mps1-3mdb cells were genetically marked and grown together with wild-type MPS1 cells. Cells were grown in complete medium in the absence (open squares) or presence (closed circles) of 6 µg/ml nocodazole for three days with dilution of the co-cultures once per day. Samples of the cultures were plated daily to determine the relative numbers of MPS1 and mps1-3mdb cells present. The markers were swapped, the experiment repeated, and the averaged results of three independent trials are shown. (F) Expression of Mps1-3mdb delays mitotic exit in the presence of mild spindle disruption. Asynchronous cells expressing endogenous MPS1 (open squares) or mps1-3mdb (closed circles) were treated with 100 ng/ml alpha factor in the absence or presence of a semi-permissive concentration of nocodazole (6 µg/ml) at time zero. G1-arrested cells were counted at 30-minute intervals. The averaged results of two trials showing the percentage of cells passing through mitosis into G1 was calculated and plotted. In (A), (E) and (F), error bars denote standard errors.