Abstract
Planning is an important aspect of many daily activities for humans. Planning involves forming a strategy in anticipation of a future need. However, evidence that nonhuman animals can plan for future situations is limited, particularly in relation to the many other kinds of cognitive capacities that they appear to share with humans. One critical aspect of planning is the ability to remember future responses, or what is called prospective coding. Two monkey species performed a series of computerized tasks that required encoding a future response at the outset of each trial. Monkeys of both species showed competence in all tests that were given, providing evidence that they anticipated future responses, and that they appropriately engaged in those responses when the time was right for such responses. In addition, some tests demonstrated that monkeys even remembered future responses that were not as presently motivating as were other aspects of the task environment. These results indicated that monkeys can anticipate future responses and retain and implement those responses when appropriate.
Keywords: Prospective coding, Planning, Monkeys, Macaca mulatta, Cebus apella
Introduction
Planning involves the development and implementation of strategies in anticipation of future needs or situations. Planning is a basic requirement of many cognitive processes employed by adult humans (Owen, 1997). Some have argued that envisioning the future and planning for it are core functions of human memory (e.g., Klein, Robertson, & Delton, 2010). With regard to the most advanced kinds of planning, it remains unclear whether animals can show similar capacities to humans, because these forms of planning seem to require mental time travel (see Roberts, 2002). Despite this potential limitation, there are some very nice demonstrations of future oriented processes in animals that might reflect planning. For example, Chappell and Kacelnik (2002) reported that crows chose appropriate tools in anticipation of future food retrieval. Similarly, chimpanzees have been observed transporting nut-cracking rocks considerable distances, suggesting that they collected those rocks with the intention of cracking and eating nuts (Boesch & Boesch, 1984).
Planning in animals is not limited to tool selection. Computerized tasks presented to nonhuman primates have shown some evidence that primates plan future responses, although in all cases this evidence is limited to only the very near future (within a few seconds). For example, various monkey species may plan travel routes through computerized mazes (Fragaszy, Johnson-Pynn, Hirsh, & Brakke, 2003; Mushiake, Saito, Sakamoto, Sato, & Tanji, 2001; Washburn, 1992). Animals also have shown evidence for planning on limited scales for sequenced responding to stimuli (e.g., Beran, Pate, Washburn, & Rumbaugh, 2002; Biro & Matsuzawa, 1999; Kawai & Matsuzawa, 2000; Scarf & Colombo, 2009, 2010).
Another capacity that seems likely to be related to planning is the use of prospective coding of future responses. Here, the idea is that an animal (or human) could encode a future response that cannot be made immediately, and then retain that intended response until later implementation. One can imagine the way this would work by thinking about the delayed matching-to-sample (DMTS) paradigm, in which a sample is presented, but then is removed for some period of time before match choice are presented. Although animals may remember, at the time of choice, what sample they had seen at the beginning of the trial (retrospective coding), they also may encode, at the beginning of the trial, what stimulus they will select later at the time of choice (prospective coding; see Roitblat, 1980). There is some debate about the nature of the memory code used during experimental tasks such as DMTS (e.g. Honig, 1978; Urcuioli & Zentall, 1992). Although most evidence suggests that retrospective codes are consistently used (e.g., Urcuioli & Zentall, 1986), there is some evidence in support of the idea of prospective coding for some tasks (e.g., Jackson-Smith, Zentall, & Steirn, 1993; Santi & Roberts, 1985; Zentall, Jagielo, Jackson-Smith, & Urcuioli, 1987).
This is true not only for matching-to-sample tests, but also for other tests such as radial arm maze tests, in which an animal can receive a reward at the end of each maze arm by running down that arm only once. In this radial maze task, one can distinguish between prospective and retrospective codes on the basis of where in a sequence of maze runs errors are most likely to occur. If errors occur most often when the maze is approximately half depleted, it would suggest that animals shift from a retrospective code early (“where have I been”) to a prospective code later (“where do I still need to go”). Some animals seem to show exactly this dissociation in coding (e.g, Cook, Brown, & Riley, 1985; Zentall, Steirn, & Jackson-Smith, 1990; but see DiGian & Zentall, 2007; Klein, Evans, & Beran, 2011).
Consider also the task used by Colombo and Graziano (1994), which used an auditory–visual matching task to investigate the type of coding used by pigeons. In this task, the sample stimuli were tones and the match stimuli were objects associated with the tones. The researchers found that interference occurred when visual stimuli were presented during the delay between sample and match presentation, but not when auditory stimuli were presented during the delay, suggesting that monkeys were using a prospective code of the anticipated visual match stimulus. This dissociation between retrospective and prospective coding may be evident in neural processes as well. Rainer, Rao, and Miller (1999) reported that monkeys showed a shift in prefrontal neural activity throughout the delay period of a delayed paired-associate task that suggested early in the trial the monkeys were remembering the sample but late in the trial were remembering the target.
We presented rhesus monkeys and capuchin monkeys with a variation of a matching-to-sample task in an effort to examine specific aspects of their behavior that would be relevant to planning behavior, at least as evidenced through use of prospective coding of responses. Here, we discuss a very limited form of planning, similar to that seen in tests of motor planning in animals (e.g., Chapman & Weiss, 2010), and some of the computerized tests that show that animals may anticipate and plan one or more responses in a sequence (e.g., Beran et al., 2002; Biro & Matsuzawa, 1999; Scarf & Colombo, 2010). Our test was based on the delayed matching-to-sample tasks conducted to examine the different processes that might occur during a retention interval. However, we devised a new variation of that test that placed a greater premium on the use of a prospective code rather than a retrospective code, to determine whether rhesus monkeys and capuchin monkeys could solve such a task through use of prospective coding of future responses.
Experiment 1
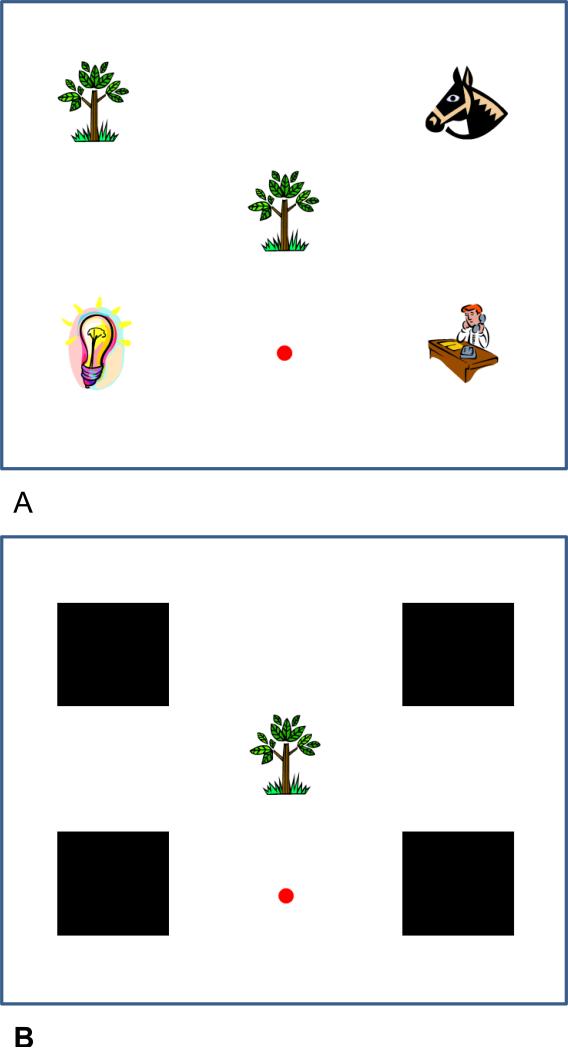
In this task, a sample image appeared in the center of the computer screen at the same time that four choice images (one match and three non-matches) appeared in the corners of the screen (see Figure 1). The monkey had to move a cursor, from a location on the edge of the screen, into contact with the sample in the center of the screen. However, by the end of training in Experiment 1, movement of the cursor toward the sample stimulus immediately concealed the four comparisons. Therefore, monkeys could only succeed on this task if they discerned the location of the correct comparison before even beginning the trial by starting toward the sample. This manipulation was critical for making this task require a prospective mode of encoding (i.e., remembering where they needed to go later) rather than a retrospective mode of coding (i.e., remembering what they had seen as the sample). In essence, monkeys had to remember not what the sample was, but what match choice they were going to choose in the (near) future.
Figure 1.
Depictions of the task. A. Monkeys initially saw a sample image in the center of the screen and up to four choice images in the corners. They had to determine where the correct match was located prior to moving the cursor into contact with the image in the center. B. When the cursor moved toward the sample, masks were placed over all choice images, so that monkeys no longer could look to find the matching image.
Methods
Participants
Eight capuchin monkeys and eight rhesus monkeys were tested. The capuchins were Drella (male, 20 years old), Gabe (male, 12 years old), Griffin (male, 13 years old), Liam (male, 7 years old), Lily (female, 13 years old), Logan (male, 5 years old), Nala (female, 8 years old), and Wren (female, 8 years old). The macaques were all adult males named Murph (17 years old), Lou (17 years old), Willie (25 years old), Hank (27 years old), Chewie (11 years old), Han (8 years old), Luke (11 years old), and Obi (7 years old). All were previously trained to use joysticks to participate in computerized tests and had years of experience performing in such tasks. Thus, they had all learned previously how to contact stimuli on the screen using the cursor shown on the screen.
Apparatus
The monkeys were tested using the Language Research Center's Computerized Test System—LRC-CTS (described in Evans, Beran, Chan, Klein, & Menzel, 2008; Rumbaugh, Richardson, Washburn, Savage-Rumbaugh, & Hopkins, 1989; Washburn & Rumbaugh, 1992)—comprising a personal computer (Systemax, Port Washington, New York), a digital joystick (Logitech Precision, ), a 17-inch LCD color monitor (Acer, San Jose, CA), and a pellet dispenser (Med Associates, St. Albans, VT). Monkeys viewed the monitor from a distance of approximately 30 to 60 cm depending on each monkey's own preference for where in the test cage it sat as it worked. Monkeys manipulated the joystick to produce isomorphic movements of a computer-graphic cursor on the screen. Contacting appropriate computer-generated stimuli with the cursor brought them a 45-mg (capuchins) or 94-mg (macaques) fruit-flavored chow pellet (Bio-Serv, Frenchtown, NJ) using a pellet dispenser interfaced to the computer through a digitial I/O board (PDISO8A; Keithley Instruments, Cleveland, OH).
Design and Procedure
At the outset of each trial, a sample image appeared in the center of the screen along with four choice stimuli around the screen edge (Figure 1a). These stimuli were clip art images (3.4 cm by 3.4 cm) randomly drawn from a library of over 500 images on each trial, and the match choices were approximately 12 cm apart from their horizontal neighbor and 7.5 cm apart from their vertical neighbor. The locations of the choice stimuli were randomly determined so that the location of the match choice that was the same as the sample varied across trials. The cursor initially appeared in any of the four positions between two adjacent match stimuli. To be successful, the monkey had to view the screen and determine the location of the correct match choice before responding, because movement of the cursor would cause the match options to become masked (Figure 1b), eliminating any future opportunity for the subject to discern the correct response. Therefore, at the outset of each trial, the subject had to remember not what the sample was, but which match choice they were going to choose later in the trial. At present, however, the monkey still had to move the cursor into contact with the sample before the trial would continue. Once the sample was contacted, it disappeared from the screen for the remainder of the trial, and the monkey had to remember the correct response location without any additional information about the identity of the sample if it was to correctly complete the matching task.
During training, there were five phases. In Phase 1, there were only two match choices. In Phase 2, this was increased to four match choices. Phase 3 to Phase 5 progressively slowed the speed of the cursor to increase the trial duration. Within each of these phases, the subject progressed through seven steps, each of which successively decreased the distance from the start position that the cursor could move before the match choices were masked. Step 1 was the easiest, and the cursor had to be in contact with the sample before the match choices were masked. By Step 7, however, any movement of the joystick led to the masks appearing. At that point, the monkey had to decide which match choice it would later choose before it initiated any cursor movement or engaged the task at all. To progress from one phase to the next, a monkey had to successfully meet criterion at all seven steps within that phase and do so within a single test session. The criterion was 20 trials correct for the most recent 25 trials at each step in all phases except Phase 5, where this was reduced to 16 trials correct out of 20 to accommodate that trials required more time to complete and that animals needed the time to complete enough trials in the session to make it through all seven steps.
Monkeys worked as many trials as they chose to in a session. Macaques had access to the task for approximately 4 hours in each test session, and capuchins had access for 2 to 3 hours in each session. Correctly completed trials were rewarded with a single food pellet. Incorrect trials led to either a 10 sec (Phase 1-3) or 5 sec (Phase 4-5) timeout period during which the screen was blank. There was a 1 sec inter-trial interval for trials that followed both correct and incorrect responses.
Results
Because of the stringent progression criterion, any monkey that met that criterion substantially exceeded chance levels of performance. Chance was 50% in Phase 1 and 25% for all subsequent phases. Completing 20 of 25 trials correctly or 16 of 20 trials correctly was significantly better than this chance level, p < .01. Here, we report in how many phases, and in how many steps within phases, each monkey met the criterion.
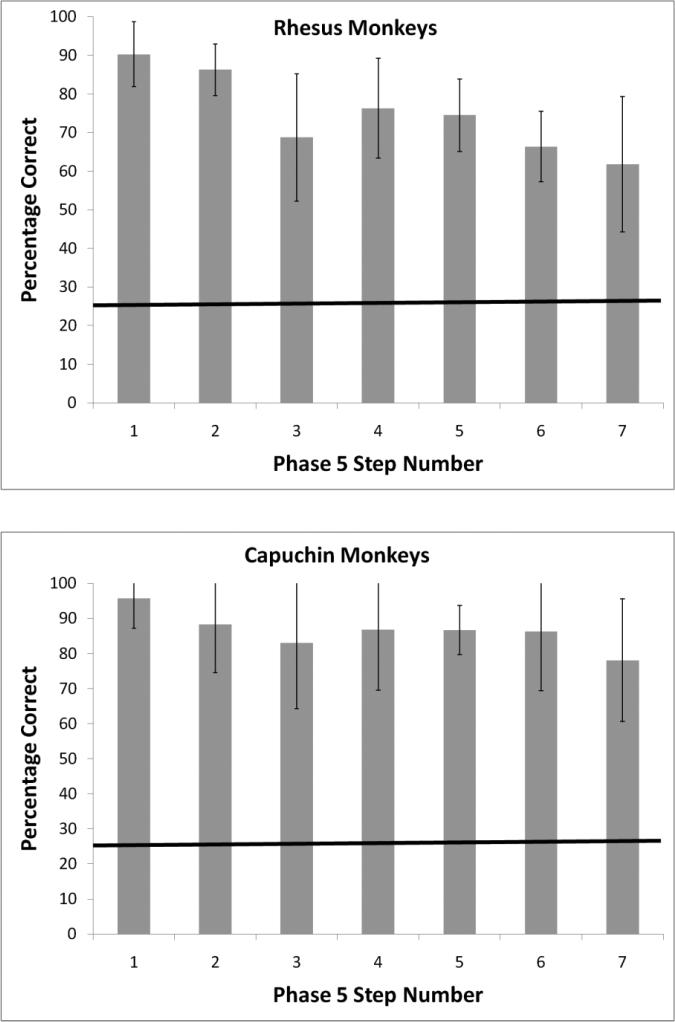
Table 1 presents the number of sessions required by each monkey at each phase to either meet the criterion or work for multiple consecutive sessions with no discernible improvement. Six of eight capuchin monkeys progressed all the way to Phase 5, and four of those six monkeys met the criterion at all seven steps within that final phase. Seven of eight rhesus monkeys also progressed all the way to Phase 5, and five of those seven monkeys met the criterion at all seven steps within that final phase. Figure 2 presents the mean performance of these four capuchin monkeys and five rhesus monkeys on their very last session in this experiment, which was the hardest test in the series. By Step 7 of Phase 5, any deflection of the joystick masked the match options, and then the monkeys had to slowly move the cursor to the sample before they were allowed to make a matching choice. The mean trial durations at this step and phase from the point at which the masks appeared onscreen to when the monkey completed its matching response were as follows (data are presented only for successful monkeys): Capuchin monkeys: Griffin – 8.3 s; Liam - 9.7 s; Logan – 12.0 s; Nala – 10.9 s; Rhesus monkeys: Han – 9.4 s; Hank – 7.6 s; Lou – 8.1 s; Murph – 10.2 s; Obi – 8.0 s.
Table 1.
Number of sessions to meet criterion for each monkey in Experiment 1
| Phase 1 | Phase 2 | Phase 3 | Phase 4 | Phase 5 | |
|---|---|---|---|---|---|
| Capuchins | |||||
| Drella | 19 | 5 | 20 | DNMC | |
| Gabe | 10 | 4 | 9 | 19 | DNMC |
| Griffin | 9 | 7 | 2 | 8 | 1 |
| Liam | 9 | 4 | 3 | 4 | 3 |
| Lily | 19 | 10 | 1 | 2 | DNMC |
| Logan | 10 | 4 | 3 | 6 | 2 |
| Nala | 10 | 4 | 3 | 2 | 6 |
| Wren | 16 | 4 | 5 | DNMC | |
| Rhesus | |||||
| Murph | 4 | 3 | 1 | 7 | 1 |
| Lou | 3 | 1 | 2 | 6 | 1 |
| Willie | 7 | 6 | 10 | 9 | DNMC |
| Hank | 5 | 4 | 12 | 4 | 5 |
| Chewie | 3 | 6 | 1 | DNMC | |
| Han | 5 | 2 | 1 | 5 | 1 |
| Luke | 2 | 3 | 4 | 4 | DNMC |
| Obi | 3 | 3 | 1 | 1 | 2 |
Note. DNMC means the monkey did not meet the criterion in that phase.
Figure 2.
Mean performance of four capuchin monkeys and five rhesus monkeys on their very last session in Experiment 1. By Step 7 of Phase 5, any deflection of the joystick masked the match options, and then the monkeys had to slowly move the cursor to the sample before they were allowed to make a matching choice. Errors bars represent 95% confidence intervals.
Discussion
The majority of monkeys completed all phases and levels, suggesting that, on a very limited temporal scale, monkeys can determine a future response before initiating a computerized trial. These monkeys had to discern where the identical match choice was located in relation to the sample in the middle of the screen. They had to do this before interacting with the task, and then they had to make an initiation response by moving to the sample before they were allowed to move on to actually select the match choice. By then, it was no longer visually on the screen. If they had not already anticipated that future response, they would perform at chance levels. However, the monkeys did anticipate that response, and then followed through with it after they moved the cursor to the sample image.
In some ways, this behavior can be called planning, in the sense that monkeys could not immediately act but instead had to encode information that would later be needed to guide future action. Thus, the present results join those from other tasks (e.g., Beran et al., 2004; Biro & Matsuawa, 1999; Scarf & Colombo, 2009) to indicate some planning competence in rhesus monkeys and capuchin monkeys. However, this task used a very short time period for trials. And, the monkeys could have visually fixated on the correct response throughout the trial, and they could have focused their attention on making that solitary matching response. To better demonstrate planning-like behavior, and prospective coding of responses, something needed to occur between encoding of the future matching response for later retrieval and actually making that response. Thus, we introduced an intervening task that would force the monkeys to disengage their visual attention from the matching location and that would also increase the trial duration. This allowed us to determine whether monkeys would still prospectively encode future responses even when they had additional task demands that were qualitatively different from the memory demand of the matching component.
Experiment 2
In Experiment 2, the program introduced a filler task between selection of the sample and match stimuli so that monkeys could not simply wait to make the matching response. Instead, the monkeys had to perform a psychomotor task in which they pursued moving targets on the screen after they had encoded the correct match choice to be made later in the trial. Only after they had completed some designated number of these target trials could they then complete the matching part of the task (by moving the cursor to the correct match). The visual tracking of moving targets decreased the chances that the monkeys could use continual rehearsal and prevented the monkeys from keeping eye gaze on the correct spatial location.
Methods
Participants and Apparatus
The same monkeys participated using the same experimental apparatus and software.
Design and Procedure
The beginning of each trial looked the same as it did in Experiment 1. A sample was centered in the middle of the screen, and there were four match choices in the corners, one of which was identical to the sample. However, an intervening task was presented after the sample was contacted with the cursor. That intervening task involved the presentation of a green circle in the center of the screen that moved in a random pattern around the center part of the screen. The monkey had to move the cursor into contact with that moving circle in order to capture it and remove it from the screen. No food pellets were given for catching the moving target during the intervening task. The target circle only moved so long as the monkey was moving its cursor, so the monkey had to engage its attention to both the cursor and the target in order to complete this intervening task. Thus, the intervening task provided a distraction to the ongoing rehearsal of the memory for the match location.
There were four phases in this experiment. In Phase 1, after the monkey contacted the moving target, it disappeared and the only things remaining on the screen were the four match locations (now masked) and the cursor. So, the monkey then could complete the matching portion of the trial, and if correct it received a single food pellet. In Phase 2, the cursor speed and the speed of the moving target were both slowed so that the intervening task was more time consuming and required greater effort on the part of the monkey. This was done to increase the delay between encoding of the correct match response location and when the monkey could actually make that response. In Phase 3, two moving stimuli had to be captured, with the second one appearing after the first was contacted and removed from the screen. This further elongated the duration of the intervening task, and in Phase 4 the monkey had to contact five of these sequentially presented moving targets during the filler task. In all phases, food reward was only presented when the matching portion of the trial was completed correctly. No food reward was given for contacting the moving targets.
Each of these phases presented five steps within each session, in which the masks over the match location appeared earlier and earlier with cursor movement toward the sample, so that by Step 5 any deflection of the cursor again immediately masked the choice options (this was the same as what was designated Step 7 in Experiment 1). Criterion for each of these steps in each of these phases was again 16 trials correct in the most recent 20. Thus, by the end of this experiment, a monkey that successfully met criterion all of the way through the experiment was completing trials where it had to find the match location before doing anything else, then move the cursor to the sample, then chase and contact five moving targets in the center of the screen, and then finally complete the matching portion of the trial correctly in order to receive a food reward.
Results
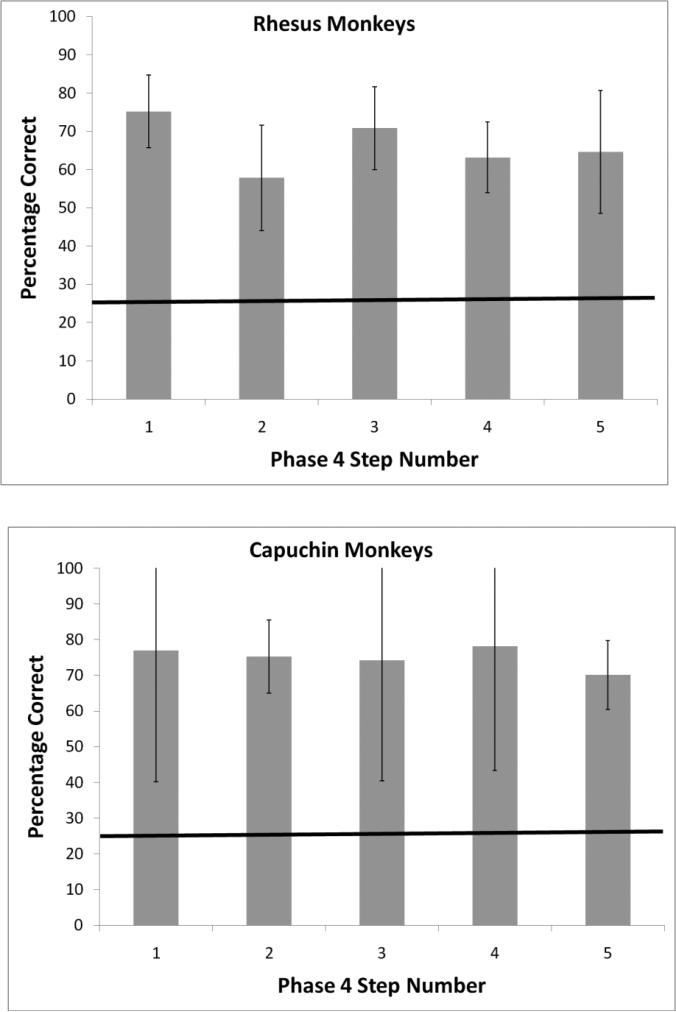
As in Experiment 1, the progression criterion meant that any monkey that met that criterion substantially exceeded chance levels of performance (p < .01, binomial test). Again, we report how far each monkey made it through the experiment and the number of sessions that each monkey completed overall in each phase (see Table 2). Six of eight capuchin monkeys progressed all the way to Phase 4, and three of those six monkeys met the criterion at all five steps within that final phase. Five of eight rhesus monkeys also progressed all the way to Phase 4, and four of those five monkeys met the criterion at all five steps within that final phase. Figure 3 presents the mean performance of these three capuchins and four rhesus monkeys on their very last session in this experiment, which was the hardest test in the series. In this last session, trial durations were longer than those of Experiment 1. The mean trial durations from the point at which the masks appeared until the monkeys made a response were as follows (data are presented only for successful monkeys): Capuchin monkeys: Liam – 18.3 s; Logan – 16.3 s; Nala – 14.0 s; Rhesus monkeys: Han – 15.5 s; Lou – 15.8 s; Murph – 17.8 s; Obi – 15.2 s.
Table 2.
Number of sessions to meet criterion for each monkey in Experiment 2
| Phase 1 | Phase 2 | Phase 3 | Phase 4 | |
|---|---|---|---|---|
| Capuchins | ||||
| Drella | 24 | DNMC | ||
| Gabe | 23 | 2 | 5 | DNMC |
| Griffin | 3 | 4 | 2 | DNMC |
| Liam | 6 | 1 | 6 | 18 |
| Lily | 25 | 2 | 1 | DNMC |
| Logan | 9 | 1 | 1 | 12 |
| Nala | 11 | 1 | 2 | 2 |
| Wren | 25 | DNMC | ||
| Rhesus | ||||
| Murph | 11 | 2 | 1 | 3 |
| Lou | 2 | 1 | 1 | 2 |
| Willie | DNMC | |||
| Hank | 1 | DNMC | ||
| Chewie | DNMC | |||
| Han | 2 | 1 | 1 | 4 |
| Luke | 1 | 1 | 1 | DNMC |
| Obi | 1 | 1 | 1 | 3 |
Note. DNMC means the monkey did not meet the criterion in that phase.
Figure 3.
Mean performance of three capuchins and four rhesus monkeys on their very last session Experiment 2. Errors bars represent 95% confidence intervals.
Discussion
That nearly half of the monkeys completed all phases and levels indicated that, on a longer temporal scale than in Experiment 1, monkeys could anticipate a future response before initiating a computerized trial. After prospectively encoding this future response, monkeys now also had to engage another task that required them to attend to other parts of the screen and perform motor responses that were in conflict with those necessary later to complete the matching part of the trial. Although this manipulation does not necessarily prevent continual rehearsal of the later matching response it certainly decreases the chances that monkeys were continuously rehearsing the matching response to be made later in the trial.
Despite the fact that all monkeys showed some success, and many monkeys completed all levels of each presented phase, it was still true that the intervening task had few motivational properties in comparison to the matching component of the test. The monkeys only received food reward for completing the matching component successfully. Thus, the intervening task was not inherently motivating except as a means to move on to the part of the test where food could be earned, and so this greatly increased the chances that the monkeys were remaining attentive to and perhaps even rehearsing the correct match location. This raised another important issue in the assessment of future-oriented processes in nonhuman animals. Specifically, the question is whether animals, like humans, can anticipate future responses that are not, in the present, highly motivating.
Some people have argued that planning in animals is limited to situations in which motivation at the time a behavior is planned matches that at the time of the planned response (sometimes this is called the Bischof-Köhler hypothesis; Bischof, 1978; Roberts, 2002; Suddendorf & Corballis, 2007; Tulving, 2005). However, some recent reports seem to suggest that animals may be capable of anticipating future needs that differ from present ones. For example, Raby, Alexis, Dickinson, and Clayton (2007) reported that scrub jays (Aphelocoma californica) would cache food in locations that were close to where the birds would be later when they were in a hungry state (see also Correia, Dickinson, & Clayton, 2007; Feeney, Roberts, & Sherry, 2011, for other positive outcomes). Mulcahy and Call (2006) reported that bonobos and orangutans transported and saved tools that they could only use 1 hour or 14 hours later. Osvath and Osvath (2008) showed that chimpanzees and orangutans could override immediate needs by choosing a tool they could only use later rather than choosing a food they could eat now. Experiment 3 attempted to manipulate motivation in a more subtle manner, but one that could provide useful data within the computerized paradigm that has been outlined
Experiment 3
In Experiment 3, we provided the monkeys with substantially more food reward for doing the filler task than they could receive for the matching response. This was designed to greatly reduce the appetitive and motivational value of making the matching response compared to the filler task. Here, the question was whether monkeys would still prospectively encode that matching response even though it provided very little value relative to the filler task in terms of the magnitude of reward that was earned. Such results would indicate that animals will encode future responses even when other things are presently more motivating in a task context. Although not a direct assessment of the Bischof-Köhler hypothesis, this manipulation seems valuable for assessing the broader issue of what constrains nonhuman animal prospective coding. It is clear that animals would be highly motivated to prospectively encode responses (if they are so capable) when those responses are the only means for obtaining food reward in experimental tasks. However, little research has asked about such encoding in a context in which the benefits for prospectively remembering future responses are much lower than the benefits for concurrent task demands, and whether this manipulation would effectively disrupt prospective coding. So, these data provide another possible route for determining how motivation interacts with memory in computerized tests and possibly other tests with concurrent task demands.
Methods
Participants and Apparatus
The same monkeys participated using the same experimental apparatus and software.
Design and Procedure
The beginning of each trial looked the same to the monkey, and, when the sample was contacted, moving targets again appeared as in Experiment 2. In the first session, monkeys had to contact five successive moving targets before moving on to the matching response. For each contacted target, the probability of getting a single food pellet was .33. A single pellet also was given for a correct matching response. At the end of the session, if the monkey exceeded 50% correct on the matching response (which exceeded chance levels of performance, p < .05, binomial test), the next session presented five additional moving targets before the match response could be made. So, monkeys would progressively complete 5, 10, 15, etc., target contacts on each trial across sessions until they reached a point at which performance did not exceed 50% correct for five consecutive sessions, at which point their participation in this experiment ended. The criterion was lowered because it was anticipated that the diminished level of reward for the matching component of trials should have some detrimental effect on performance, as would the progressively longer trial durations that would occur as monkeys were presented with higher numbers of moving targets before they could attempt the matching response.
Again, moving target contacts were rewarded on a .33 probability schedule. This meant that, for example, trials with 15 moving targets would produce, on average, 5 food pellets for the monkey before the matching component of the trial could be completed. Thus, monkeys that could increase their number of moving target contacts by meeting the session criterion would be getting more food for completing the intervening task than for completing the matching task. Unlike previous experiments, different levels of cursor movement before masks appeared over the choice options were not used. Instead, an intermediate step was used where masks appeared when the cursor was halfway between its start point and the sample image.
Results
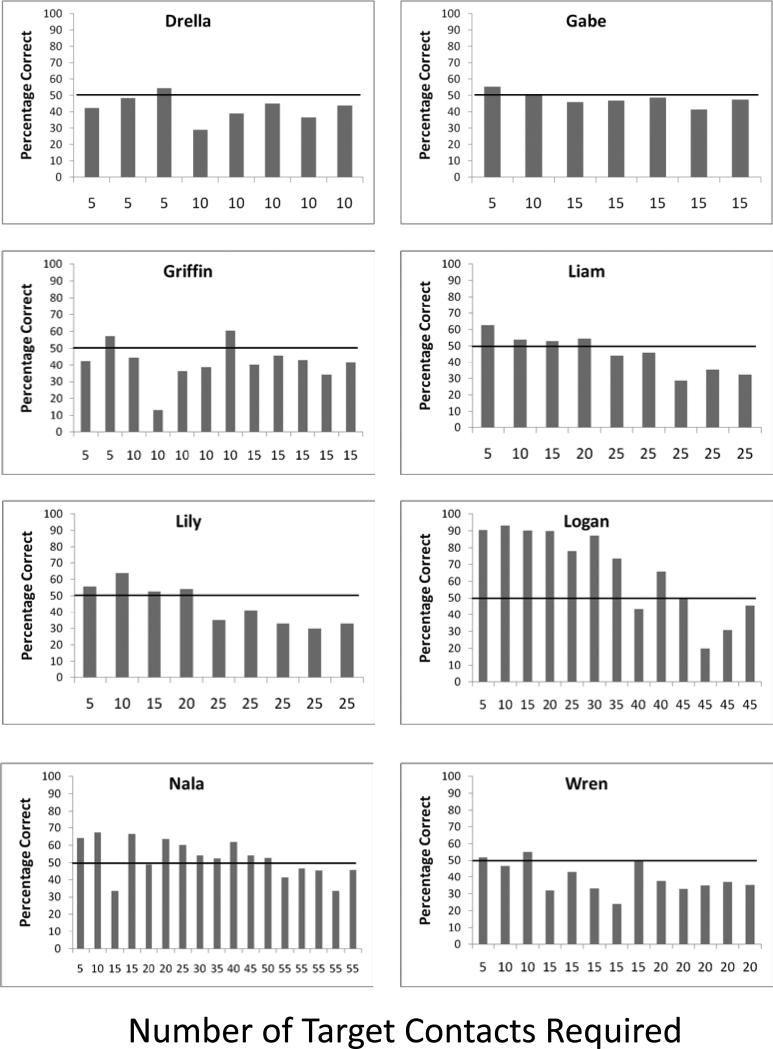
Figure 4 (capuchin monkeys) and Figure 5 (rhesus monkeys) show the session by session performance level of each monkey for each number of moving target contacts that were required before a matching response could be made. Capuchin monkeys were successful, with all individuals meeting criterion for at least the 5-contact trials, and four of eight monkeys meeting criterion for 20-contact trials or better. The average trial durations for the last test session at which criterion was met for each of these four monkeys, from the point at which the masks appeared until the match response was made, were as follows: Liam – 68.2 s; Lily – 55.2 s; Logan – 112.9 s; Nala – 123.6 s. Two monkeys in particular stand out. Logan met criterion even when completing 40 contacts with moving targets, and Nala did so for trials with 50 such contacts. This means that on average Logan and Nala were receiving 13.3 and 16.7 pellets per trial for contacting moving targets for every possible single-pellet reward in the matching component of the task.
Figure 4.
Session by session performance level of each capuchin monkey for each number of moving target contacts that were required before a matching response could be made in Experiment 3. The horizontal line indicates the 50% performance level that was the criterion for moving to a larger number of required target contacts. Chance was still 25% as in the previous experiments.
Figure 5.
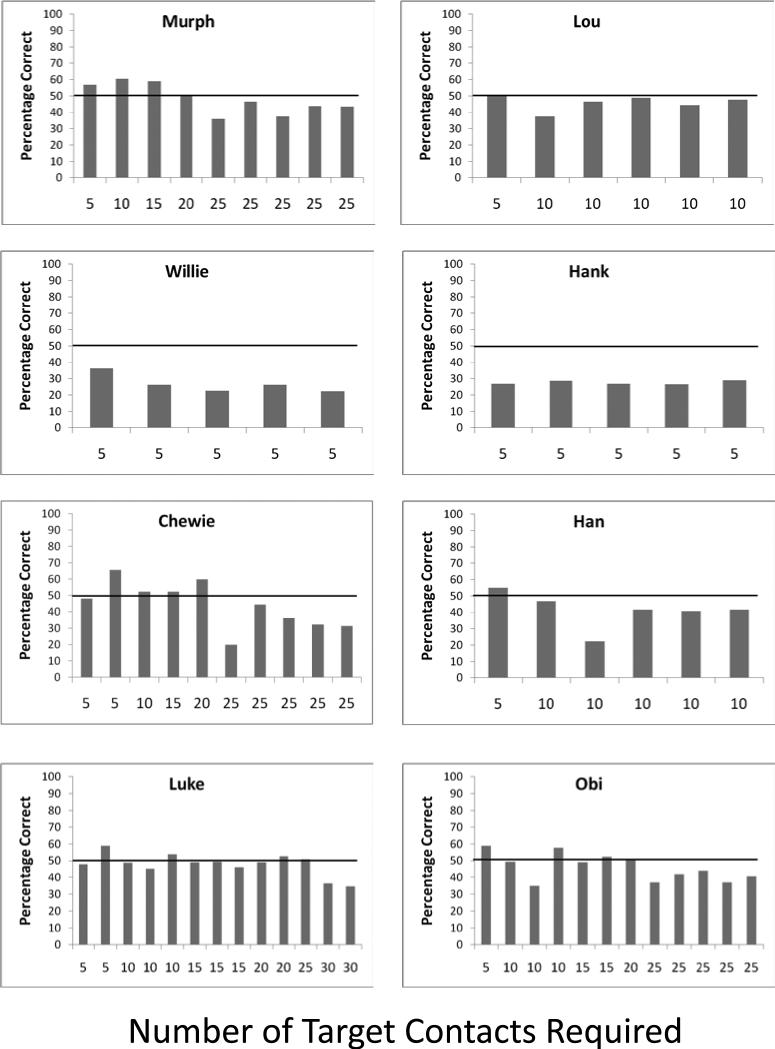
Session by session performance level of each rhesus monkey for each number of moving target contacts that were required before a matching response could be made in Experiment 3. The horizontal line indicates the 50% performance level that was the criterion for moving to a larger number of required target contacts. Chance was 25%.
Rhesus monkeys also showed success. All rhesus monkeys except for one met criterion for at least the 5-contact trials, and four of eight monkeys met criterion for 20-contact trials. The average trial durations for the last test session at which criterion was met for each of these four monkeys, from the point at which the masks appeared until the match response was made, were as follows: Chewie – 65.5 s; Luke – 70.6 s; Murph – 51.5 s; Obi – 48.7 s.
Discussion
These results indicate that the majority of monkeys performed well by encoding a future response even when they were more heavily rewarded on the intervening task. This should have greatly decreased the immediate motivation for the planned matching response encoded at the outset of the trial. By this we mean that monkeys learned that the target tracking component of the task was much more heavily rewarded. This result is important for what it might indicate about the potential for nonhuman animals to plan future responses. Planning involves the development and implementation of strategies in anticipation of future needs or situations. As noted earlier, the Bischof-Köhler hypothesis states that animals cannot plan for future rewards that are not presently desired. Although the present results do not refute this hypothesis, it should be clear that performance on the matching component of the trial was greatly diminished in its motivational structure compared to performance on the target-contacting component because of the greatly inflated number of pellets that could be obtained in the latter component relative to the former. Yet, most monkeys still encoded that future spatial response location and retained that information even while completing, in some cases, dozens of target contacts and eating in some cases 10 times as many pellets during that part of each trial. This indicates that monkeys will encode future responses even when those responses are not the most presently motivating features of their test environment, including here when successful performance on the matching component made only a small contribution to the overall food consumption of the monkeys.
General Discussion
This series of experiments progressively increased the difficulty of a matching-to-sample task that was designed to probe potential planning and prospective encoding of responses. Monkeys of two species succeeded in all tests presented to them in this study, illustrating a number of points about the planning and memory capacities of nonhuman animals. In particular, four aspects of this project merit attention.
First, as demonstrated initially in Experiment 1, but also throughout the entire study, all monkeys were capable of forming an intended future response before initiating a trial. Experiment 1 required by its end that a monkey survey the computer screen, encode where the correct match choice was located, and then remember that location for at least as long as it took to move the cursor first to the sample in the center of the screen. By the end of Experiment 1, monkeys had to do this before they engaged the joystick or moved the cursor, and so their success indicated they inhibited that initiation of the joystick long enough to accurately assess what response they would make. In this sense, the present data support other reports that monkeys can plan responses on a computer screen, at least to a limited temporal degree (e.g., Beran et al., 2004, Scarf & Colombo, 2009, 2010, 2011).
Second, the monkeys demonstrated that not only would they encode this future response when they were preparing to make it in the very near future (i.e., when there was nothing else to do but make that response), but they also proved capable of doing that even when an intervening task was introduced. That task prevented continuous visual fixation because the monkeys instead had to track moving targets and move the cursor in directions not directly related to the later matching response. Monkeys did this quite well, in part because matching performance still dictated whether the monkeys received any food reward. On the other hand, the large number of trials in each session surely could have produced high levels of proactive interference, and it is impressive that the monkeys were able to plan a response and then execute it after delays filled with an intervening activity. Thus, forming a durable encoding of a future response and then performing an intervening task before being allowed to make that response is within the capacity of monkeys of both species, as clearly indicated in Experiment 2. The present task extends previous tests of planning using computerized tests by adding this intervening task requirement, and monkeys still succeed.
Third, the monkeys’ performance provided evidence contrary to one of the main issues that is foundational to the Bischof-Köhler hypothesis – that animals do not anticipate future responses that are less motivating than possible responses in the present. We made the intervening task substantially more motivating in terms of food reward that could be obtained. Thus, matching responses in Experiment 3 were reduced in their importance compared to Experiment 1 and Experiment 2. But, most monkeys demonstrated that they still would encode and remember the correct match location before obtaining most of their food reward from the intervening task. Some monkeys, in fact, showed extremely high levels of performance in terms of how many target contacts they could perform and still show high matching performance. Thus, Experiment 3 demonstrated that monkeys can encode future responses and later execute them even when those responses are not the most motivating aspects of the animals’ present environment. This does not refute the Bischof-Köhler hypothesis, which is more specific with regard to its claim that animals cannot plan for things they presently do not want, because in our task, pellets were all equally motivating. However, we contend that these data still are interesting because they address the issue of relative motivational value of different task components in a multi-response test paradigm, and the data suggest monkeys will engage in a concurrent task to obtain a relatively small reward amount even when that concurrent task is the harder component of an ongoing experiment (e.g., spatial memory encoding and retention versus psychomotor responding). Moreover, the results demonstrate that monkeys can plan for actions that will not benefit them until 60 s or so later.
A reviewer of this paper suggested that the monkeys could retrospectively retrieve memories of the sample and memories of the stimuli originally shown in each match location for that trial. Then, monkeys could have found the equivalent match choice for that sample, and completed the trial, without need of encoding any future response at any point in the trial. Although this possibility cannot be fully discounted with the present data, we believe that such a strategy or response pattern would be much less efficient and require higher memory loads than the prospective coding interpretation. Monkeys would have to store more information at the trial outset – the sample, and each of the four choice stimuli as well as their locations. Then, at test, the monkeys would have to compare all of those stored units of information to find the matching pair, before moving to the location of that matching item. Given the large number of trials and the potentially high levels of interference across trials from using a retrospective coding strategy, it seems unlikely that this strategy (without a deliberate attempt to encode the location of the objects) could support such high levels of performance.
Planning has been reserved by some as a uniquely human capacity. However, nonhuman animals have shown some planning-like behavior, albeit in far more restricted ways than for other behaviors shared with humans. The present experiments suggest that two species of monkeys, an Old World species and a New World species, may, in a self-initiated manner, plan future responses on a limited time scale. In addition, the matching to sample task used here has clear applications for assessing deterioration of planning and prospective encoding and memory as a function of age, cognitive load (e.g., by making the intervening task more cognitively demanding), and other variables that can account for individual differences. As such, it is a useful tool for better understanding the evolution of future oriented processes in nonhuman animals and may provide even greater insights into the future oriented processes that are present in nonhuman animals.
The ability to anticipate and plan for the future also factors heavily into specialized kinds of memory such as prospective memory. Prospective memory refers to remembering to perform some action in the future, and it relies on the processes of encoding, storing, and delayed retrieval of a future action (e.g., Einstein & McDaniel, 1990, 2005; Ellis, 1996; Ellis & Freeman, 2008; Kliegel, McDaniel, and Einstein, 2000; Shallice & Burgess, 1991). Humans are quite capable at using prospective memory for a variety of tasks on a wide range of time scales, from remembering something in the immediate future (e.g., to attach a file to an email message one is about to send) to the more distant future (e.g., to change the oil in one's car next weekend; Kliegel et al., 2000; McDaniel & Einstein, 2007; McDaniel, Einstein, Graham, & Rall, 2004; Smith, 2003, 2008). Some of those tasks may even approximate the demands of the computer task used here – for example, having to remember to specifically encode where you parked your car in a complex airport parking lot because you will need that information later. In order to do this, one has to be aware that this information will be necessary in the future and one has to self-initiate the encoding process. Thus, experimental paradigms that can illustrate some of the constituent processes of prospective memory in animals can have value for better understanding similar mechanisms in humans. Although the present task cannot assess prospective memory in animals, adaptations of that paradigm that allow animals to choose when to report remembered responses on the basis of either temporal or event-based cues will provide additional data pertaining to future oriented processes in nonhuman animals.
Acknowledgments
This research was supported by grants HD38051, HD060563, and HD061455 from the National Institute of Child Health and Human Development and by grants BCS-0634662 and BCS-0924811 from the National Science Foundation.
The authors thank Betty Chan, Dan Rice, and Timothy Flemming for assistance with data collection.
Contributor Information
Michael J. Beran, Language Research Center, Georgia State University, University Plaza, Atlanta, GA 30302; mjberan@yahoo.com.
Theodore A. Evans, Language Research Center, Georgia State University
Emily D. Klein, Language Research Center, Georgia State University
Gilles O. Einstein, Department of Psychology, Furman University.
References
- Beran MJ, Pate JL, Washburn DA, Rumbaugh DM. Sequential responding and planning in chimpanzees (Pan troglodytes) and rhesus macaques (Macaca mulatta). Journal of Experimental Psychology: Animal Behavior Processes. 2004;30:203–212. doi: 10.1037/0097-7403.30.3.203. [DOI] [PubMed] [Google Scholar]
- Biro D, Matsuzawa T. Numerical ordering in a chimpanzee (Pan troglodytes): Planning, executing, and monitoring. Journal of Comparative Psychology. 1999;113:178–185. [Google Scholar]
- Bischof N. On the phylogeny of human morality. In: Stent G, editor. Morality as a biological phenomenon. Abakon; Berlin: 1978. pp. 53–74. [Google Scholar]
- Boesch C, Boesch H. Mental map in wild chimpanzees: An analysis of hammer transports for nut cracking. Primates. 1984;25:160–170. [Google Scholar]
- Chapman KM, Weiss DJ, Rosenbaum DA. Evolutionary roots of motor planning: The end-state comfort effect in lemurs. Journal of Comparative Psychology. 2010;124:229–232. doi: 10.1037/a0018025. [DOI] [PubMed] [Google Scholar]
- Chappell J, Kacelnik A. Tool selectivity in a non-primate, the New Caladonian crow (Corvus moneduloides). Animal Cognition. 2002;5:71–78. doi: 10.1007/s10071-002-0130-2. [DOI] [PubMed] [Google Scholar]
- Colombo M, Graziano M. Effects of auditory and visual interference on auditory-visual delaying matching to sample in monkeys (Macaca fascicularis). Behavioral Neuroscience. 1994;108:636–639. doi: 10.1037//0735-7044.108.3.636. [DOI] [PubMed] [Google Scholar]
- Cook RG, Brown MF, Riley DA. Flexible memory processing by rats: Use of prospective and retrospective information in tha radial maze. Journal of Experimental Psychology: Animal Behavior Processes. 1985;11:453–469. [PubMed] [Google Scholar]
- Correia SPC, Dickinson A, Clayton NS. Western scrub-jays anticipate future needs independently of their current motivational state. Current Biology. 2007;17:856–861. doi: 10.1016/j.cub.2007.03.063. [DOI] [PubMed] [Google Scholar]
- DiGian KA, Zentall TR. Pigeons may not use dual coding in the radial maze analog task. Journal of Experimental Psychology: Animal Behavior Processes. 2007;33:262–272. doi: 10.1037/0097-7403.33.3.262. [DOI] [PubMed] [Google Scholar]
- Einstein GO, McDaniel MA. Normal aging and prospective memory. Journal of Experimental Psychology: Learning, Memory, and Cognition. 1990;16:716–726. doi: 10.1037//0278-7393.16.4.717. [DOI] [PubMed] [Google Scholar]
- Einstein GO, McDaniel MA. Prospective memory: Multiple retrieval processes. Current Directions in Psychological Science. 2005;14:286–290. [Google Scholar]
- Ellis JA. Prospective memory or the realization of delayed intentions: A conceptual framework for research. In: Brandimonte M, Einstein GO, McDaniel MA, editors. Prospective memory: Theory and applications. Lawrence Erlbaum; Mahwah, NJ: 1996. pp. 1–22. [Google Scholar]
- Ellis JA, Freeman JE. Realizing delayed intentions. In: Kliegel M, McDaniel MA, Einstein GO, editors. Prospective memory: Cognitive, neuroscience, developmental and applied perspectives. Lawrence Erlbaum; New York: 2008. pp. 1–27. [Google Scholar]
- Evans TA, Beran MJ, Chan B, Klein ED, Menzel CR. An efficient computerized testing method for the capuchin monkey (Cebus apella): Adaptation of the LRC-CTS to a socially housed nonhuman primate species. Behavior Research Methods. 2008;40:590–596. doi: 10.3758/brm.40.2.590. [DOI] [PubMed] [Google Scholar]
- Feeney MC, Roberts WA, Sherry DF. Black-capped chickadees (Poecile atricapillus) anticipate future outcomes of foraging choices. Journal of Experimental Psychology: Animal Behavior Processes. 2011;37:30–40. doi: 10.1037/a0019908. [DOI] [PubMed] [Google Scholar]
- Fragaszy D, Johnson-Pynn J, Hirsh E, Brakke K. Strategic navigation of two-dimensional alley mazes: Comparing capuchin monkeys and chimpanzees. Animal Cognition. 2003;6:149–160. doi: 10.1007/s10071-002-0137-8. [DOI] [PubMed] [Google Scholar]
- Honig WK. Studies of working memory in the pigeon. In: Hulse SH, Fowler H, Honig WW, editors. Cognitive processes in animal behavior. Erlbaum; Hillsdale, NJ: 1978. pp. 211–248. [Google Scholar]
- Jackson-Smith P, Zentall TR, Steirn JN. Prospective and retrospective memory processes in pigeons’ performance on a successive delayed matching-to-sample task. Learning & Motivation. 1993;24:1–22. [Google Scholar]
- Kawai N, Matsuzawa T. Numerical memory span in a chimpanzee. Nature. 2000;403:39–40. doi: 10.1038/47405. [DOI] [PubMed] [Google Scholar]
- Klein ED, Evans TA, Beran MJ. An investigation of prospective and retrospective coding in capuchin monkeys and rhesus monkeys. Zeitschrift für Psychologie. 2011;219:85–91. doi: 10.1027/2151-2604/a000052. [Google Scholar]
- Klein SB, Robertson TE, Delton AW. Facing the future: Memory as an evolved system for planning future acts. Memory & Cognition. 2010;38:13–22. doi: 10.3758/MC.38.1.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kliegel M, McDaniel MA, Einstein GO. Plan formation, retention, and execution in prospective memory: A new approach and age related effects. Memory and Cognition. 2000;28:1041–1049. doi: 10.3758/bf03209352. [DOI] [PubMed] [Google Scholar]
- McDaniel MA, Einstein GO. Prospective memory. Sage Publications; Los Angeles: 2007. [Google Scholar]
- McDaniel MA, Einstein GO, Graham T, Rall E. Delaying execution of intentions: Overcoming the costs of interruptions. Applied Cognitive Psychology. 2004;18:533–547. [Google Scholar]
- Mulcahy NJ, Call J. Apes save tools for future use. Science. 2006 May 19;312:1038–1040. doi: 10.1126/science.1125456. [DOI] [PubMed] [Google Scholar]
- Mushiake H, Saito N, Sakamoto K, Sato Y, Tanji J. Visually based path-planning by Japanese monkeys. Cognitive Brain Research. 2001;11:165–169. doi: 10.1016/s0926-6410(00)00067-7. [DOI] [PubMed] [Google Scholar]
- Osvath M, Osvath H. Chimpanzee (Pan troglodytes) and orangutan (Pongo abelii) forethought: Self-control and pre-experience in the face of future tool use. Animal Cognition. 2008;11:661–674. doi: 10.1007/s10071-008-0157-0. [DOI] [PubMed] [Google Scholar]
- Owen AM. Cognitive planning in humans: Neuropsychological, neuroanatomical, and neuropharmacological perspectives. Progress in Neurobiology. 1997;53:431–450. doi: 10.1016/s0301-0082(97)00042-7. [DOI] [PubMed] [Google Scholar]
- Raby CR, Alexis DM, Dickinson A, Clayton NS. Planning for the future by western scrub-jays. Nature. 2007;445:919–921. doi: 10.1038/nature05575. [DOI] [PubMed] [Google Scholar]
- Rainer G, Rao SC, Miller EK. Prospective coding for objects in primate prefrontal cortex. The Journal of Neuroscience. 1999;19:5493–5505. doi: 10.1523/JNEUROSCI.19-13-05493.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts WA. Are animals stuck in time? Psychological Bulletin. 2002;128:473–489. doi: 10.1037/0033-2909.128.3.473. [DOI] [PubMed] [Google Scholar]
- Roitblat HL. Codes and coding processes in pigeon short-term memory. Animal Learning and Behavior. 1980;8:341–351. [Google Scholar]
- Rumbaugh DM, Richardson WK, Washburn DA, Savage-Rumbaugh ES, Hopkins WD. Rhesus monkeys (Macaca mulatta), video tasks, and implications for stimulus-response spatial contiguity. Journal of Comparative Psychology. 1989;103:32–38. doi: 10.1037/0735-7036.103.1.32. [DOI] [PubMed] [Google Scholar]
- Santi A, Roberts WA. Prospective representation: The effects of varied mapping of sample stimuli to comparison stimuli and differential trial outcomes on pigeons’ working memory. Animal Learning and Behavior. 1985;13:103–108. [Google Scholar]
- Scarf D, Colombo M. Eye movements during list execution reveal no planning in monkeys (Macaca fascicularis). Journal of Experimental Pscyhology. 2009;35:587–592. doi: 10.1037/a0014020. [DOI] [PubMed] [Google Scholar]
- Scarf D, Colombo M. The formation and execution of sequential plans in pigeons (Columba livia) Behavioural Processes. 2010;83:179–182. doi: 10.1016/j.beproc.2009.12.004. [DOI] [PubMed] [Google Scholar]
- Scarf D, Danly E, Morgan G, Colombo M, Terrace HS. Sequential planning in rhesus monkeys (Macaca mulatta). Animal Cognition. 2011;14(3):317–324. doi: 10.1007/s10071-010-0365-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shallice T, Burgess P. Deficits in strategy application following frontal lobe damage in man. Brain. 1991;114:727–741. doi: 10.1093/brain/114.2.727. [DOI] [PubMed] [Google Scholar]
- Smith RE. The cost of remembering to remember in event-based prospective memory: Investigating the capacity demands of delayed intention performance. Journal of Experimental Psychology: Learning, Memory, and Cognition. 2003;29:347–361. doi: 10.1037/0278-7393.29.3.347. [DOI] [PubMed] [Google Scholar]
- Smith RE. Attention, memory, and delayed intentions. In: Kliegel M, McDaniel MA, Einstein GO, editors. Prospective memory: Cognitive, neuroscience, developmental and applied perspectives. Lawrence Erlbaum; New York: 2008. pp. 29–52. [Google Scholar]
- Suddendorf T, Corballis MC. The evolution of foresight: What is mental time travel, and is it unique to humans? Behavioral and Brain Sciences. 2007;30:299–351. doi: 10.1017/S0140525X07001975. [DOI] [PubMed] [Google Scholar]
- Tulving E. Episodic memory and autonoesis: Uniquely human? In: Terrace H, Metcalfe J, editors. The missing link in cognition: Evolution of self-knowing consciousness. Oxford University Press; New York: 2005. pp. 3–56. [Google Scholar]
- Urcuioli P, Zentall TR. Retrospective coding in pigeons’ delayed matching-to-sample. Journal of Experimental Psychology: Animal Behavior Processes. 1986;12:69–77. [PubMed] [Google Scholar]
- Urcuioli PJ, Zentall TR. Transfer across delayed discriminations: Evidence regarding the nature of prospective working memory. Journal of Experimental Psychology: Animal Behavior Processes. 1992;18:154–173. doi: 10.1037//0097-7403.18.2.154. [DOI] [PubMed] [Google Scholar]
- Washburn DA. Analyzing the path of responding in maze-solving and other tasks. Behavior, Research Methods, Instruments, and Computers. 1992;24:248–252. doi: 10.3758/bf03203502. [DOI] [PubMed] [Google Scholar]
- Washburn DA, Rumbaugh DM. Testing primates with joystick-based automated apparatus: Lessons from the Language Research Center's Computerized Test System. Behavior Research Methods, Instruments, & Computers. 1992;24:157–164. doi: 10.3758/bf03203490. [DOI] [PubMed] [Google Scholar]
- Zentall TR, Jagielo JA, Jackson-Smith P, Urcuioli PJ. Memory codes in pigeons’ short-term memory: Effects of varying the number of sample and comparison stimuli. Learning & Motivation. 1987;18:21–33. [Google Scholar]
- Zentall TR, Steirn JN, Jackson-Smith P. Memory strategies in pigeons’ performance of a radial-arm-maze analog task. Journal of Experimental Psychology: Animal Behavior Processes Processes. 1990;20:390–402. [Google Scholar]