Abstract
Objective
Diosmin, a natural flavone glycoside, possesses antioxidant activity and has been used to alleviate ischemia/reperfusion (I/R) injury. The aim of this study was to clarify whether the administration of diosmin has a protective effect against I/R injury induced using the high intraocular pressure (IOP) model in rat retina, and to determine the possible antioxidant mechanisms involved.
Methods
Retinal I/R injury was induced in the rats by elevating the IOP to 110 mmHg for 60 min. Diosmin (100 mg/kg) or vehicle solution was administered intragastrically 30 min before the onset of ischemia and then daily after I/R injury until the animals were sacrificed. The levels of malondialdehyde (MDA) and the activities of total-superoxide dismutase (T-SOD), glutathione peroxidase (GSH-Px), and catalase (CAT) in the retinal tissues were determined 24 h after I/R injury. At 7 days post-I/R injury, electroretinograms (ERGs) were recorded, and the density of surviving retinal ganglion cells (RGCs) was estimated by counting retrograde tracer-labeled cells in whole-mounted retinas. Retinal histological changes were also examined and quantified using light microscopy.
Results
Diosmin significantly decreased the MDA levels and increased the activities of T-SOD, GSH-Px, and CAT in the retina of rats compared with the ischemia group (P<0.05), and suppressed the I/R-induced reduction in the a- and b-wave amplitudes of the ERG (P<0.05). The thickness of the entire retina, inner nuclear layer, inner plexiform layer, and outer retinal layer and the number of cells in the ganglion cell layer were significantly less after I/R injury (P<0.05), and diosmin remarkably ameliorated these changes on retinal morphology. Diosmin also attenuated the I/R-induced loss of RGCs of the rat retina (P<0.05).
Conclusion
Diosmin protected the retina from I/R injury, possibly via a mechanism involving the regulation of oxidative parameters.
Introduction
A number of ocular diseases have been associated with retinal ischemia/reperfusion (I/R) injury, including retinal vascular occlusion, acute glaucoma, diabetic retinopathy, and retinopathy of prematurity.1 I/R injury leads to energy-dependent dysfunction, tissue edema, and, eventually, to retinal cell death. Nevertheless, the exact mechanism of cell death induced by retinal ischemia is not completely understood. Endogenous excitatory amino acids (e.g., glutamate), oxygen-free radicals, nitric oxide, and calcium imbalances are involved in the pathogenesis of I/R injury.2,3 Of these mechanisms, oxidative stress, which may occur because of an imbalance between the production and removal of reactive oxygen species (ROS), is considered to be a critical mediator of retinal ganglion cell (RGC) injury of various etiologies and has been implicated in RGC death.4
The generation of excess ROS can affect subcellular structures and functions, resulting in mitochondrial dysfunction, lipid peroxidation of polyunsaturated fatty acids in membranes, and alterations in protein or DNA structure.5 There is an endogenous free-radical scavenging system6; however, excess ROS can overwhelm this system and contribute directly to cell damage. In ischemia followed by reoxygenation during reperfusion, ROS are overproduced at a level that exceeds the clearance ability of the body. This contributes to cell death directly via the damage of cellular components and indirectly by modulating the signaling pathways involved in the cell death process.7
A number of in vivo studies have reported a protective effect of antioxidants in retinas after I/R-induced damage.4,8–10 A powerful free-radical scavenger,11 diosmin is a natural flavone glycoside readily obtained by dehydrogenation of the corresponding flavanone glycoside hesperidin; it is also abundant in the pericarp of various citrus fruits.12,13 Diosmin has been reported to be effective in the prevention of I/R-induced intestinal injury, and in tissue damage in the kidney, liver, intestine, cheek pouch, and brain.14–18 However, there are few studies18 on the protective effect of diosmin against retinal damage caused by I/R.
In the present study, we investigated the effect of the intragastric administration of diosmin (standardized up to 95% diosmin) on rat retina after injury using the high intraocular pressure (IOP) model. We also examined the changes in retinal tissues associated with oxidative stress, including changes in the levels of malondialdehyde (MDA), total-superoxide dismutase (T-SOD), glutathione peroxidase (GSH-Px), and catalase (CAT), to elucidate the mechanism by which diosmin protects retinal neurons. Our findings indicate that diosmin protects retinal neurons from oxidative stress, and that it can improve both morphological damage and functional changes after retinal I/R injury.
Methods
Rat model of acute elevated IOP
Healthy male Wistar rats (n=112) weighing 180–200 g each were purchased from the Shanghai Laboratory Animal Center of the Chinese Academy of Sciences. The animals used in this study were treated in accordance with the Association for Research in Vision and Ophthalmology (ARVO) Statement for the Use of Animals in Ophthalmic and Vision Research. The animals were housed under controlled conditions that included a 12-h light/dark cycle (8:00–20:00 h light; 20:00–8:00 h dark), a temperature of 23°C–25°C, humidity in the range of 55%–60%, and with free access to standard food and drinking water.
All surgeries were performed under aseptic conditions. The rats were anesthetized by an intraperitoneal (i.p.) injection of 1% pentobarbital sodium (10 mg/kg). Corneal analgesia was achieved using 1 or 2 drops of 0.4% oxybuprocaine hydrochloride, pupillary dilatation was maintained with 0.5% tropicamide and 0.5% phenylephrine, and the body temperature was maintained at 36.5°C–37°C using a heating blanket. After dilation of the pupil, the anterior chamber of the right eye was cannulated with a 30-gauge needle connected to a physiological saline reservoir. The IOP was raised to 110 mmHg by keeping the reservoir at 150 cm above the eye, and retinal ischemia was confirmed by examination of the fundus. After 60 min, the IOP was returned to normal pressure by removing the infusion needle from the anterior chamber. Ofloxacin ophthalmic gel (0.3%) was applied topically to the eye before and after the procedure. Only the right eye was used in all experiments. The anterior chamber of the right eye in the 2 sham-operated groups was similarly cannulated without raising the IOP.
Group assignment and drug administration
For intragastric administration, diosmin (D3525; Sigma-Aldrich, St. Louis, MO) was diluted in physiological saline, the vehicle solution. Next, 5 mL of 2% diosmin per kilogram weight of the rat, or the same volume of vehicle solution, was administered intragastrically 30 min before the onset of ischemia, and then daily after I/R injury until the animals were sacrificed.
The animals were randomly assigned to the following 4 groups, which included combinations of the I/R injury model or sham injury with the i.g. administration of diosmin or vehicle solution: sham+vehicle (SV) group, sham+diosmin (SD) group, model+vehicle (MV) group, and model+diosmin (MD) group.
Using an overdose of anesthesia, 8 rats from each group were sacrificed 24 h after I/R injury, and their eyeballs harvested for determination of the MDA level and the activities of T-SOD, GSH-Px, and CAT. At 7 days post-I/R injury, electroretinograms (ERGs) were recorded in 6 rats per group. Meanwhile, 6 rats in each group were randomly chosen for retrograde labeling of RGCs, and the remaining 8 rats from each group were histopathologically examined.
Determination of the levels of MDA and activities of T-SOD, GSH-Px, and CAT
The levels of MDA and the activities of T-SOD, GSH-Px, and CAT were determined in each retina 24 h after the ischemic insult, as described previously.19 The retina samples were prepared as a 10% homogenate in 0.9% saline using a homogenizer on ice according to their respective weight. Next, the homogenate was centrifuged at 2,000 rpm for 10 min, and the supernatant was collected and diluted. MDA levels were determined using the double heating method. Briefly, 2.5 mL of thiobarbituric acid (TBA) means thiobarbituric acid solution (100 g/L) was added to 0.5 mL of homogenate in each centrifuge tube, and the tubes were placed in boiling water for 15 min. After cooling with flowing water, the tubes were centrifuged at 1,000 rpm for 10 min, 2 mL of the supernatant was added to 1 mL of the TBA solution (6.7 g/L), and the tube was placed in boiling water for another 15 min. After cooling, the amount of thiobarbituric acid-reactive species was measured at 532 nm. The MDA concentration was calculated from the absorbance coefficient of the MDA-TBA complex. The data are expressed as nM/g of wet tissue. T-SOD activity was determined through the inhibition of nitrotetrazolium blue (NTB) reduction by the xanthine/xanthine oxidase system as a superoxide generator. The activity was assessed in the supernatant after the addition of 1.0 mL of ethanol/chloroform (5/3, v/v) to the same volume of sample and centrifugation for 15 min at 3,000 rpm. The production of formazan was determined at 560 nm. One unit of SOD activity was defined as the amount of protein that inhibited the rate of NTB reduction by 50%. The data are expressed as U/g of protein. GSH-Px activity was measured by the method of Paglia and Valentine.20 The enzymatic reaction in the tube that contained reduced nicotinamide adenine dinucleotide phosphate, reduced glutathione, sodium azide, and glutathione reductase was initiated by the addition of H2O2, and the change in absorbance at 340 nm was monitored by spectrophotometry. The data are expressed as U/g of protein. CAT activity was measured by the method of Cohen.21 The principle of the assay was based on the determination of the rate constant (s−1, k) of H2O2 decomposition. The rate constant of the enzyme was determined by measuring the absorbance change per minute. The data are expressed as k/g of protein.
Electroretinography
At 7 days after I/R injury, the retinas of rats from each group were assessed using ERGs (Tomey EP-1000, Erlangen, Germany). After overnight dark adaptation, the rats were anesthetized under dim red illumination and their pupils dilated with 0.5% tropicamide and 0.5% phenylephrine. The rats were placed in a stereotaxic frame, lying on an electric blanket to maintain the body temperature at 37°C, and facing the stimulus at a distance of 20 cm. Stainless steel wire (0.1 mm in diameter) loops were placed on the cornea to act as the recording electrode after the topical application of ofloxacin ophthalmic gel. A reference electrode was connected to the middle of the lower eyelid together with a grounding electrode near the tail. The responses to a light flash (2.5 cd·s/m2) from a photic stimulator were amplified, and the preamplifier bandwidth was set at 0.3–300 Hz. The amplitude of the a-wave was measured from the baseline to the trough of the a-wave, while that of the b-wave was measured from the trough of the a-wave to the peak of the b-wave.
Retrograde labeling of RGCs
We identified RGCs by retrograde labeling with fluorogold (FG) dye (Biotium, Hayward, CA), using a slight modification of the method of Peinado-Ramon et al.22 Briefly, 7 days before sacrifice, the animals were deeply anesthetized by an i.p. injection of 1% pentobarbital sodium and placed in stereotactic apparatus (Stoelting, Kiel, Germany). The head was then immobilized and the scalp incised. Located by the lambda and bregma sutures, 2 small holes (1.5×1.5 mm) were drilled into the pericranium, and the neuronal tracer FG was injected (8 μL of 4% FG in distilled water) into the bilateral superior colliculis (SC, 6.0 mm posterior to the bregma, 1.2 mm lateral, and a depth of 3.7 mm), the main targets of rat RGC projections. To prevent FG diffusion along the needle track, the injection needle was not withdrawn from the brain until 2 min after injection. After wound closure with a suture, the rats were allowed to recover on a heating blanket that maintained their body temperature at 37°C.
At 7 days after I/R injury, the animals were sacrificed by an overdose of 3% pentobarbital sodium. Immediately after sacrifice, the eyes of each rat were enucleated and fixed in 4% paraformaldehyde for 1 h. Then, the posterior segments were prepared for flat mounts after removal of the lens and the vitreous body. Retinal tissue was carefully placed on a nitrocellulose membrane, with the ganglion cell layer on top. The density of FG-positive RGCs was determined by fluorescence microscopy (Zeiss 510, Jena, Germany) in a masked fashion, as described previously.23 Briefly, we photographed 3 standard rectangular areas (0.2×0.2 mm; 0.04 mm2) at 1, 2, and 3 mm, respectively, from the optic disc in the central region of each retinal quadrant (superotemporal, inferotemporal, superonasal, and inferonasal). Thus, for each retina, 12 rectangles were evaluated, for a total area of 0.48 mm2 (12×0.04 mm2), which is ∼1% of the total area of the retina,24 assuming an average area of 50 mm.2 To determine the density of FG-positive RGCs as cells/mm,2 we multiplied the number of cells per 0.04 mm2 by 25. The data are given as the mean RGCs/mm2±standard deviation.
Histopathologic examination
At 7 days postretinal I/R injury, the eyeballs were enucleated and fixed in 4% paraformaldehyde at 4°C for 24 h. After fixation, the anterior segment was removed, and the posterior eyeball was dehydrated in a graded ethanol series and embedded in paraffin. For hematoxylin and eosin (HE) staining, samples were cut into 5-μm-thick retinal cross sections and observed under a light microscope (Leica, Heidelberg, Germany). To quantify the ischemic damage in the retina, we measured different layer thicknesses to quantify the degree of cell loss. The overall retinal thicknesses (from the inner limiting membrane to the pigment epithelium), the outer retinal layers (ORL, consisting of the outer nuclear and outer plexiform layers), the inner nuclear layer (INL), and the inner plexiform layer (IPL) were measured. All measurements (400×) were made ∼2 to 3 disc diameters from the optic disc. The number of cells in the ganglion cell layer (GCL) was calculated using the linear cell density (cells per 200 μm), and 3 sections per eye were averaged.
Statistical analysis
All of the results are expressed as the mean±standard deviation. Statistical analyses were performed using SPSS for Windows version 17.0 (SPSS, Inc., Chicago, IL). To identify significant differences, an analysis of variance and the Student-Newman-Keuls test were applied. P values less than 0.05 were considered to be statistically significant.
Results
Effect of diosmin on the MDA level and activity of T-SOD, GSH-Px, and CAT
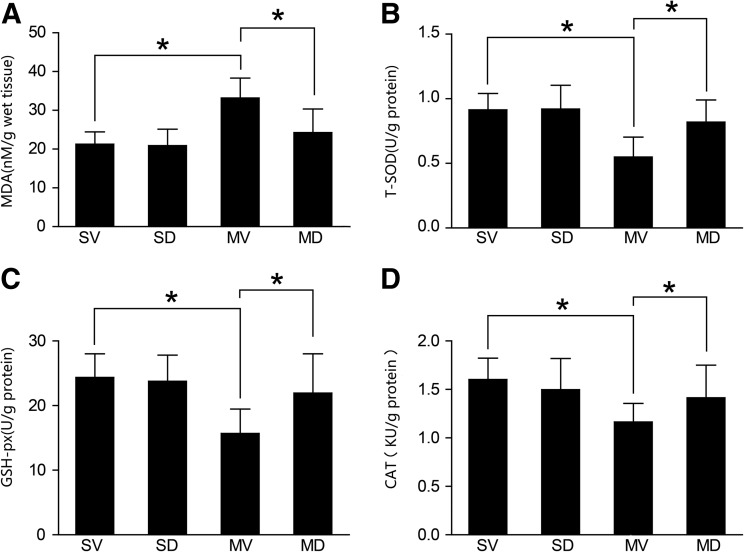
Twenty-four hours after retinal I/R injury, the MDA levels in the MV group were significantly higher than that in the other 3 groups. However, compared with the MV group, diosmin significantly reduced the MDA levels in the MD group (21.27±3.16, 20.89±4.27, 33.17±5.14, and 24.29±6.08 nM/g of wet tissue for the SV, SD, MV, and MD group, respectively; n=8) (P<0.05, Fig. 1A). The activity levels of T-SOD (0.914±0.127, 0.921±0.183, 0.549±0.154, and 0.819±0.172 U/g of protein for the SV, SD, MV, and MD groups, respectively; n=8), GSH-Px (24.36±3.64, 23.77±4.05, 15.69±3.78, and 21.94±6.07 U/g of protein for the SV, SD, MV, and MD groups, respectively; n=8), and CAT (1.603±0.219, 1.498±0.321, 1.164±0.191, and 1.414±0.337 KU/g of protein for the SV, SD, MV, and MD groups, respectively; n=8) were similar between the SV and SD groups (P>0.05, Fig. 1B–D), and they were significantly higher than those in the MV group (P<0.05, Fig. 1B–D). Compared with the MV group, diosmin significantly increased the activities of T-SOD, GSH-Px, and CAT in the MD group (P<0.05, Fig. 1B–D).
FIG. 1.
Changes in the level of MDA and the activities of T-SOD, GSH-Px, and CAT in the retina 24 h after I/R (n=8). The level of MDA in the MV group was significantly higher than that in both SV and SD groups (P<0.05); the level of MDA in the MD group was significantly lower than that in the MV group (P<0.05) (A). The activities of T-SOD, GSH-Px, and CAT in the MV group were significantly lower than those in both SV and SD groups (P<0.05); the activities in the MD group was significantly higher than those in the MV group (P<0.05) (B–D). *P<0.05. I/R, ischemia/reperfusion; MDA, malondialdehyde; T-SOD, total-superoxide dismutase; GSH-Px, glutathione peroxidase; CAT, catalase; SV, sham+vehicle; MV, model+vehicle; MD, model+diosmin; SD, sham+diosmin.
Effect of diosmin on RGC survival
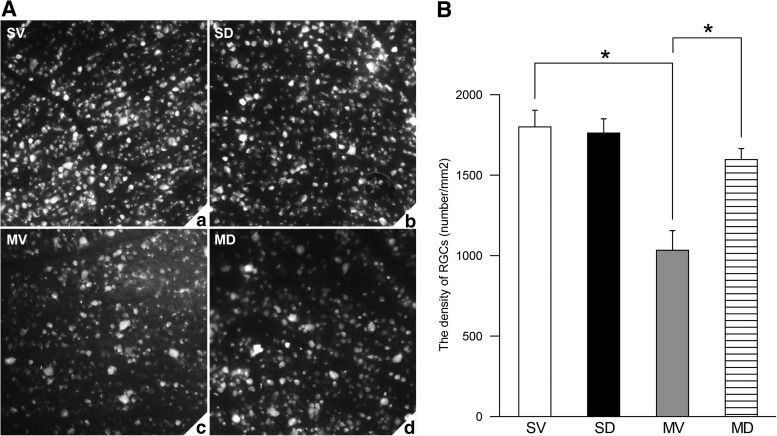
Only surviving RGCs can be labeled with FG. As shown in Fig. 2A, at 7 days after I/R injury, there was a remarkable loss of RGCs in the MV group compared with the SV and SD groups; however, diosmin significantly attenuated this reduction. The density of surviving RGCs was 1,801±103/mm2 (n=6) in the SV group and 1,763±88/mm2 (n=6) in the SD group (P>0.05, Fig. 2B). The finding that the density of surviving RGCs in the MV group was reduced by 43% compared with that in the SV group (1,034±122 vs. 1,801±103/mm2; n=6) (P<0.05, Fig. 2B) reflects the fact that I/R insult could result in RGC death. However, the density of surviving RGCs in the MD group (1,597±69/mm2; n=6) was significantly higher than that in the MV group (P<0.05, Fig. 2B) and recovered to 89% when compared with the SV group, showing the preventive effect of diosmin on the loss of RGCs after I/R insult.
FIG. 2.
Representative photographs of retrogradely labeled retinas in the 4 groups (A). The density of surviving RGCs in the MV group was significantly lower than that in both SV and SD groups (P<0.05) (n=6) (B); the density of surviving RGCs in the MD group was significantly higher than that in the MV group (P<0.05) (n=6) (B). *P<0.05. RGCs, retinal ganglion cells.
Preventive effect of diosmin on the reduction in amplitude of a- and b-waves
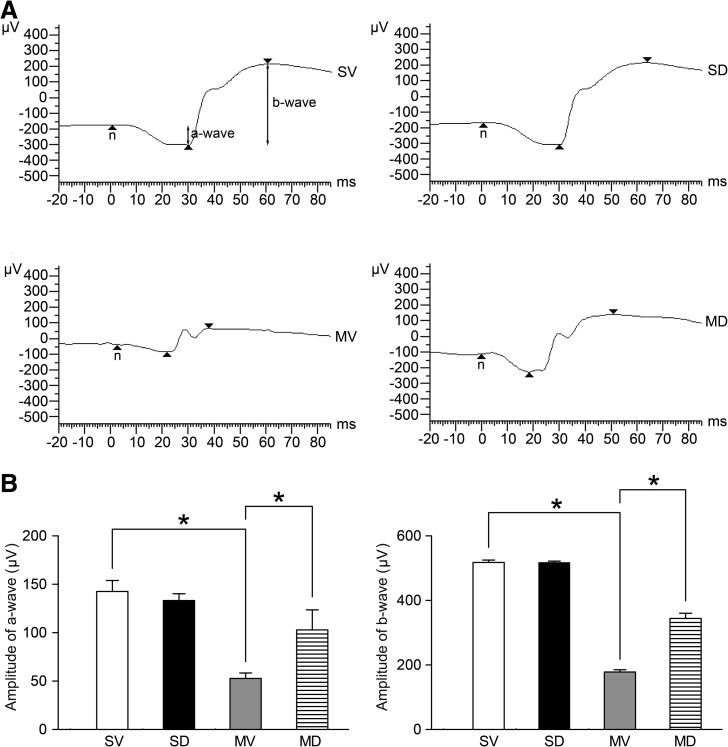
To evaluate the functional changes induced by I/R injury, an ERG was recorded on day 7 after I/R injury. Figure 3A shows typical ERGs for the 4 groups, 7 days after I/R injury. The finding that the a- and b-wave amplitudes in the MV group was reduced by 63% and 66%, respectively, compared with that in the SV group (52.83±5.65 vs. 142.50±11.40 μV for a-wave and 178.34±6.74 vs. 517.86±7.31 μV for b-wave; n=6) (P<0.05, Fig. 3B) reflected the fact that the I/R insult could result in retinal functional impairment. However, the a- and b-wave amplitudes in the MD group (102.99±20.52 μV for a-wave and 344.36±15.73 μV for b-wave; n=6) were significantly higher than those in the MV group (P<0.05, Fig. 3B) and recovered to 72% and 66%, respectively, when compared with the SV group, showing the preventive effect of diosmin on the retinal functional impairment after I/R insult.
FIG. 3.
Representative photographs of ERG records in the 4 groups (A). The responses to a light flash (2.5 cds/m2) from a photic stimulator were amplified, and the pre-amplifier bandwidth was set at 0.3–300 Hz. The ERG was recorded 7 days after I/R injury, and the a- and b-wave amplitudes were measured. Data are expressed as the mean±standard deviation (n=6) (B). *P<0.05. ERG, electroretinogram.
Effect of diosmin on retinal damage
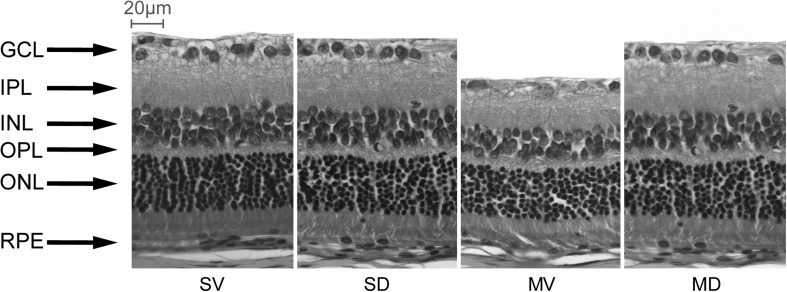
At 7 days post-IR injury, the morphological changes were evaluated. The retinas from the SV and SD groups were normal in appearance (Fig. 4). Compared with the SV and SD groups, the thickness of the retina in the MV group was significantly decreased, and the reduction was primarily attributed to significant degeneration of the cell bodies in the GCL and thinning of the INL, IPL, and ORL (Fig. 4). The overall retinal thickness in the MV group was reduced by 24% compared with that in the SV group (87.84±7.27 vs. 116.27±5.14 μm) (P<0.05, Table 1). The retinal layers, in order of reduced magnitude, were INL, IPL, and ORL, which were reduced by 38, 32, and 18%, respectively; the GCL density was reduced by 55% in the SV group (Fig. 4 and Table 1). In contrast, diosmin clearly protected against the retinal ischemic damage in the MD group, with normal patterns of organization in the outer nuclear and plexiform layers, except for a slight misalignment of the photoreceptors (Fig. 4). The thicknesses of the inner and outer retina and the GCL density in the MD group were significantly greater than those in the MV group (P<0.05, Table 1), confirming the protective effect of diosmin.
FIG. 4.
Representative photographs of the rat retinas in the 4 groups 7 days after I/R (n=8). In both SV and SD groups, the GCL and INL were obvious and well organized. Seven days after I/R, the INL in the MV group was obviously thinner, and the number of GCL cells was significantly decreased, whereas in the MD group, the retina was more normal in structure, with a thicker INL than in the MV group. GCL, ganglion cell layer; INL, inner nuclear layer; IPL, inner plexiform layer; OPL, outer plexiform layer; ONL, outer nuclear layer. Scale bar=20 μm.
Table 1.
Thickness of the Retinal Layers and GCL Cell Count at 7 Days After Ischemia/Reperfusion (Mean±Standard Deviation, n=8)
| |
Thickness (μm) |
|
|||
|---|---|---|---|---|---|
| Group (each n=8) | Overall | INL | IPL | ORL | Density of the survival of RGCs (n/200 μm) |
| SV | 116.27±5.14 | 23.79±1.64 | 31.94±2.01 | 36.27±2.19 | 18.79±0.92 |
| SD | 113.75±5.37 | 23.86±1.57 | 30.67±2.17 | 34.59±2.87 | 19.02±1.03 |
| MV | 87.84±7.27a,b | 14.65±1.71a,b | 21.58±3.31a,b | 29.87±3.01a,b | 8.47±0.89a,b |
| MD | 109.34±6.93b,c | 20.96±1.98b,c | 27.64±4.07b,c | 33.54±2.95b,c | 13.27±1.78b,c |
Compared with the SV group.
P<0.05.
Compared with the MV group.
INL, inner nuclear layer; IPL, inner plexiform layer; ORL, outer retinal layers; RGCs, retinal ganglion cells; SV, sham+vehicle; MV, model+vehicle; MD, model+diosmin; SD, sham+diosmin.
Discussion
This study evaluated the protective effect of diosmin on retinal damage caused by I/R injury in a rat model. Our results demonstrated a reduction in both morphological damage and functional changes in the MD group in comparison with the MV group. In the MD group, diosmin markedly protected the retina from I/R-induced injury by preventing the loss of retinal neurons and improving the oxidative parameters of the tissue, as evidenced by reduced levels of MDA and increased activity levels of T-SOD, GSH-Px, and CAT, suggesting that the mechanism of protection by diosmin in the retina includes a reduction in oxidative stress.
In this study, the IOP was elevated above the systolic blood pressure for 60 min, and during this period the blood supply to the retina was drastically reduced, as indicated by whitening of the fundus. Acutely raising the IOP of a rat eye, followed by reperfusion, is known to cause physiologic dysfunction.25 Therefore, we measured the ERG a- and b-wave amplitudes to evaluate the extent of functional retinal damage and to observe the morphological changes to determine the histopathological insult after I/R injury. Electroretinography is a sensitive physiological measure of retinal health: the a-wave provides information about the photoreceptors, representing the outer layer of the retina, while the b-wave provides information about the physiology of the ON bipolar and Müller cells, representing the inner layer of the retina.1,26 Therefore, the results of this study demonstrate the protective effect of diosmin on retinal cells during I/R injury. Furthermore, a good correlation was noted between the ERG for both a- and b-waves and the histological changes. After I/R injury, the reduction in the b-wave was greater than that in the a-wave, and consistent with the morphological changes shown by HE staining, the decrease in thickness of the inner layer exceeded that of the outer layer.
It has been suggested that both apoptosis and necrosis are involved in I/R-induced neuronal damage.27–29 RGCs are vulnerable to retinal I/R injury, and the numbers of surviving RGCs and their axons are important determinants of the extent of visual impairment in patients with ischemic ocular disorders, including glaucoma, diabetic retinopathy, retinal vein occlusion, and retinopathy of prematurity. Therefore, to investigate whether diosmin can prevent the loss of RGCs, we used the retrograde labeling of RGCs to quantitatively determine the number of surviving RGCs after retinal I/R injury. Consistent with some previous studies,23,30 our results show that the I/R injury induced by the acutely elevated IOP caused the loss of RGCs, and that the intragastric administration of diosmin protected the RGCs.
I/R injury involves many mechanisms that ultimately result in necrotic and apoptotic cell death.31 When the IOP is increased, glutamate is released from the retina during and after ischemia.2,32,33 The major causes of cell death after activation of the N-methyl-D-aspartate subtype of glutamate receptors are related to an influx of calcium and the generation of free radicals.34 In living organisms, free radicals are considered to be a double-edged sword. At ordinary concentrations, free radicals are essential participants in cell signaling and regulation; however, an imbalance between the production and removal of free radicals can lead to oxidative stress and subsequent cellular toxicity.35 In particular, the cellular damage that occurs secondarily to ischemia may be exacerbated by the sudden reintroduction of oxygen into tissues during reperfusion, triggering free-radical cascades that overwhelm endogenous free-radical scavengers.36 Numerous studies of retinal tissue have suggested that oxidative stress is an essential factor in cellular damage during I/R injury.4,37,38 MDA is a naturally occurring product of lipid peroxidation, a process in which unsaturated fatty acids are oxidized to form radicals. Compared with lipid peroxidation, MDA has a longer life span and is more favorable for the evaluation of the severity of tissue damage.39 Therefore, in the present study, we determined the level of MDA to assess oxidative tissue damage after I/R injury. Free radicals produced in living organisms can be scavenged by intrinsic antioxidant enzymes, including SOD, CAT, and GSH-Px. In addition, previous studies have proven that SOD and CAT administration can protect the retina from I/R injury.9,40 Therefore, the activities of these enzymes may reflect the antioxidative ability of the body. In this study, as shown in our previous reports,19,41 I/R injury resulted in a significant increase in the MDA level and decrease in the activities of T-SOD, CAT, and GSH-Px in the retina. However, in this study, we found that diosmin significantly decreased the MDA level and enhanced the antioxidant enzyme activity in retinal tissue, implying that diosmin ameliorated the injury, as assessed by the morphology and function of the retina, by attenuating the oxidative stress induced by I/R injury.
Diosmin, a naturally occurring flavonoid glycoside that can be isolated from various plant sources or derived from the flavonoid hesperidin, is a vascular-protective agent used to treat chronic venous insufficiency, hemorrhoids, lymphedema, and varicose veins. Like other flavonoids, diosmin is capable of scavenging free radicals. In the present study, we showed the protective effect of diosmin against morphological and functional damage to the retina after I/R insult, possibly based on its ability to regulate oxidative parameters.
As noted in previous studies, the antioxidative stress effect of diosmin may not be its sole protective mechanism. Various other mechanisms, including anti-inflammatory activity, which have been demonstrated in other tissues and organs,42,43 could also play a role in the protective effects observed in this study. Additional investigations are needed to clarify this issue.
In conclusion, our present study demonstrates that diosmin is effective at protecting retinal neurons during retinal I/R and improving I/R-induced retinal dysfunction. The protective effect of diosmin seems to be at least partly due to regulating the oxidative parameters. Diosmin may be a potential neuroprotective drug for retinal diseases associated with I/R injury.
Acknowledgments
The authors would like to thank Yan Xu, Jun Li, and Kai Dong for excellent technical assistance throughout the project. This work was supported by the Key Program of National Administration of Traditional Chinese Medicine, Shanghai, China (Grant No. 20062001), and Opening Project of Shanghai Key Laboratory of Fundus Diseases (Grant No. 07Z22911).
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Osborne N.N. Casson R.J. Wood J.P. Chidlow G. Graham M. Melena J. Retinal ischemia: mechanisms of damage and potential therapeutic strategies. Prog. Retin Eye Res. 2004;23:91–147. doi: 10.1016/j.preteyeres.2003.12.001. [DOI] [PubMed] [Google Scholar]
- 2.Louzada-Junior P. Dias J.J. Santos W.F. Lachat J.J. Bradford H.F. Coutinho-Netto J. Glutamate release in experimental ischaemia of the retina: an approach using microdialysis. J. Neurochem. 1992;59:358–363. doi: 10.1111/j.1471-4159.1992.tb08912.x. [DOI] [PubMed] [Google Scholar]
- 3.Dreyer E.B. A proposed role for excitotoxicity in glaucoma. J. Glaucoma. 1998;7:62–67. [PubMed] [Google Scholar]
- 4.Li S.Y. Fu Z.J. Ma H. Jang W.C. So K.F. Wong D. Lo A.C. Effect of lutein on retinal neurons and oxidative stress in a model of acute retinal ischemia/reperfusion. Invest. Ophthalmol. Vis. Sci. 2009;50:836–843. doi: 10.1167/iovs.08-2310. [DOI] [PubMed] [Google Scholar]
- 5.Zimmerman B.J. Granger D.N. Oxygen free radicals and the gastrointestinal tract: role in ischemia-reperfusion injury. Hepatogastroenterology. 1994;41:337–342. [PubMed] [Google Scholar]
- 6.Machlin L.J. Bendich A. Free radical tissue damage: protective role of antioxidant nutrients. FASEB J. 1987;1:441–445. [PubMed] [Google Scholar]
- 7.Rodrigo R. Gonzalez J. Paoletto F. The role of oxidative stress in the pathophysiology of hypertension. Hypertens. Res. 2011;34:431–440. doi: 10.1038/hr.2010.264. [DOI] [PubMed] [Google Scholar]
- 8.Demir T. Ulas F. Ozercan I. Ilhan N. Celiker U. Yasar M.A. Protective effects of pentoxifylline in retinal ischemia/reperfusion injury. Ophthalmologica. 2003;217:337–341. doi: 10.1159/000071348. [DOI] [PubMed] [Google Scholar]
- 9.Chen B. Tang L. Protective effects of catalase on retinal ischemia/reperfusion injury in rats. Exp. Eye Res. 2011;93:599–606. doi: 10.1016/j.exer.2011.07.007. [DOI] [PubMed] [Google Scholar]
- 10.Chao H.M. Lin D.E. Chang Y. Hsu W.M. Lee S.M. Lee F.L. Chi C.W. Pan W.H. Liu T.Y. Lui W.Y. Ho L.T. Kuo C.D. Chan C.C. Chao F.P. Ferulic acid, but not tetramethylpyrazine, significantly attenuates retinal ischemia/reperfusion-induced alterations by acting as a hydroxyl radical scavenger. J. Ocul. Pharmacol. Ther. 2008;24:461–472. doi: 10.1089/jop.2008.0005. [DOI] [PubMed] [Google Scholar]
- 11.Firuzi O. Miri R. Tavakkoli M. Saso L. Antioxidant therapy: current status and future prospects. Curr. Med. Chem. 2011;18:3871–3888. doi: 10.2174/092986711803414368. [DOI] [PubMed] [Google Scholar]
- 12.Campanero M.A. Escolar M. Perez G. Garcia-Quetglas E. Sadaba B. Azanza J.R. Simultaneous determination of diosmin and diosmetin in human plasma by ion trap liquid chromatography-atmospheric pressure chemical ionization tandem mass spectrometry: application to a clinical pharmacokinetic study. J. Pharm. Biomed. Anal. 2010;51:875–881. doi: 10.1016/j.jpba.2009.09.012. [DOI] [PubMed] [Google Scholar]
- 13.Nogata Y. Sakamoto K. Shiratsuchi H. Ishii T. Yano M. Ohta H. Flavonoid composition of fruit tissues of citrus species. Biosci. Biotechnol. Biochem. 2006;70:178–192. doi: 10.1271/bbb.70.178. [DOI] [PubMed] [Google Scholar]
- 14.Unlu A. Sucu N. Tamer L. Coskun B. Yucebilgic G. Ercan B. Gul A. Dikmengil M. Atik U. Effects of Daflon on oxidative stress induced by hindlimb ischemia/reperfusion. Pharmacol. Res. 2003;48:11–15. [PubMed] [Google Scholar]
- 15.Tanrikulu Y. Kismet K. Serin Kilicoglu S. Devrim E. Erel S. Sen Tanrikulu C. Dinc S. Edebal O.H. Erdemli E. Akkus M.A. Diosmin ameliorates intestinal injury induced by hepatic ischemia reperfusion in rats. Bratisl Lek. Listy. 2011;112:545–551. [PubMed] [Google Scholar]
- 16.Pehlivan M. Hazinedaroglu S.M. Kayaoglu H.A. Erkek A.B. Keklik T. Canbolat O. Kocak S. The effect of diosmin hesperidin on intestinal ischaemia—reperfusion injury. Acta Chir. Belg. 2004;104:715–718. doi: 10.1080/00015458.2004.11679649. [DOI] [PubMed] [Google Scholar]
- 17.Bouskela E. Cyrino F.Z. Lerond L. Microvascular reactivity after ischemia/reperfusion in the hamster cheek pouch: beneficial effects of different oral doses of S-5682 (Daflon 500 mg) Angiology. 1997;48:33–37. doi: 10.1177/000331979704800106. [DOI] [PubMed] [Google Scholar]
- 18.Delbarre B. Delbarre G. Calinon F. Effect of Daflon 500 mg, a flavonoid drug, on neurological signs, levels of free radicals and electroretinogram in the gerbil after ischemia-reperfusion injury. Int. J. Microcirc. Clin. Exp. 1995;15(Suppl 1):27–33. doi: 10.1159/000179092. [DOI] [PubMed] [Google Scholar]
- 19.Shi Y. Wu X. Gong Y. Qiu Y. Zhang H. Huang Z. Su K. Protective effects of caffeic acid phenethyl ester on retinal ischemia/reperfusion injury in rats. Curr. Eye Res. 2010;35:930–937. doi: 10.3109/02713683.2010.494820. [DOI] [PubMed] [Google Scholar]
- 20.Paglia D.E. Valentine W.N. Studies on the quantitative and qualitative characterization of erythrocyte glutathione peroxidase. J. Lab. Clin. Med. 1967;70:158–169. [PubMed] [Google Scholar]
- 21.Cohen G. Dembiec D. Marcus J. Measurement of catalase activity in tissue extracts. Anal. Biochem. 1970;34:30–38. doi: 10.1016/0003-2697(70)90083-7. [DOI] [PubMed] [Google Scholar]
- 22.Peinado-Ramon P. Salvador M. Villegas-Perez M.P. Vidal-Sanz M. Effects of axotomy and intraocular administration of NT-4, NT-3, and brain-derived neurotrophic factor on the survival of adult rat retinal ganglion cells. A quantitative in vivo study. Invest. Ophthalmol. Vis. Sci. 1996;37:489–500. [PubMed] [Google Scholar]
- 23.Zhang Z. Tong N. Gong Y. Qiu Q. Yin L. Lv X. Wu X. Valproate protects the retina from endoplasmic reticulum stress-induced apoptosis after ischemia-reperfusion injury. Neurosci. Lett. 2011;504:88–92. doi: 10.1016/j.neulet.2011.09.003. [DOI] [PubMed] [Google Scholar]
- 24.Crosson C.E. Mani S.K. Husain S. Alsarraf O. Menick D.R. Inhibition of histone deacetylase protects the retina from ischemic injury. Invest. Ophthalmol. Vis. Sci. 2010;51:3639–3645. doi: 10.1167/iovs.09-4538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hughes W.F. Quantitation of ischemic damage in the rat retina. Exp. Eye Res. 1991;53:573–582. doi: 10.1016/0014-4835(91)90215-z. [DOI] [PubMed] [Google Scholar]
- 26.Stockton R.A. Slaughter M.M. B-wave of the electroretinogram. A reflection of ON bipolar cell activity. J. Gen. Physiol. 1989;93:101–122. doi: 10.1085/jgp.93.1.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ettaiche M. Fillacier K. Widmann C. Heurteaux C. Lazdunski M. Riluzole improves functional recovery after ischemia in the rat retina. Invest. Ophthalmol. Vis. Sci. 1999;40:729–736. [PubMed] [Google Scholar]
- 28.Katai N. Yoshimura N. Apoptotic retinal neuronal death by ischemia-reperfusion is executed by two distinct caspase family proteases. Invest. Ophthalmol. Vis. Sci. 1999;40:2697–2705. [PubMed] [Google Scholar]
- 29.Kuroiwa S. Katai N. Yoshimura N. A possible role for p16INK4 in neuronal cell death after retinal ischemia-reperfusion injury. Invest. Ophthalmol. Vis. Sci. 1999;40:528–533. [PubMed] [Google Scholar]
- 30.Zhong Y.S. Liu X.H. Cheng Y. Min Y.J. Erythropoietin with retrobulbar administration protects retinal ganglion cells from acute elevated intraocular pressure in rats. J. Ocul. Pharmacol. Ther. 2008;24:453–459. doi: 10.1089/jop.2008.0021. [DOI] [PubMed] [Google Scholar]
- 31.Buchi E.R. Cell death in the rat retina after a pressure-induced ischaemia-reperfusion insult: an electron microscopic study. I. Ganglion cell layer and inner nuclear layer. Exp. Eye Res. 1992;55:605–613. doi: 10.1016/s0014-4835(05)80173-3. [DOI] [PubMed] [Google Scholar]
- 32.Hirooka K. Miyamoto O. Jinming P. Du Y. Itano T. Baba T. Tokuda M. Shiraga F. Neuroprotective effects of D-allose against retinal ischemia-reperfusion injury. Invest. Ophthalmol. Vis. Sci. 2006;47:1653–1657. doi: 10.1167/iovs.05-1018. [DOI] [PubMed] [Google Scholar]
- 33.Adachi K. Kashii S. Masai H. Ueda M. Morizane C. Kaneda K. Kume T. Akaike A. Honda Y. Mechanism of the pathogenesis of glutamate neurotoxicity in retinal ischemia. Graefes Arch. Clin. Exp. Ophthalmol. 1998;236:766–774. doi: 10.1007/s004170050156. [DOI] [PubMed] [Google Scholar]
- 34.Osborne N.N. Ugarte M. Chao M. Chidlow G. Bae J.H. Wood J.P. Nash M.S. Neuroprotection in relation to retinal ischemia and relevance to glaucoma. Surv. Ophthalmol. 1999;43(Suppl 1):S102–S128. doi: 10.1016/s0039-6257(99)00044-2. [DOI] [PubMed] [Google Scholar]
- 35.Droge W. Free radicals in the physiological control of cell function. Physiol. Rev. 2002;82:47–95. doi: 10.1152/physrev.00018.2001. [DOI] [PubMed] [Google Scholar]
- 36.Cuzzocrea S. Riley D.P. Caputi A.P. Salvemini D. Antioxidant therapy: a new pharmacological approach in shock, inflammation, and ischemia/reperfusion injury. Pharmacol. Rev. 2001;53:135–159. [PubMed] [Google Scholar]
- 37.Rego A.C. Santos M.S. Oliveira C.R. Influence of the antioxidants vitamin E and idebenone on retinal cell injury mediated by chemical ischemia, hypoglycemia, or oxidative stress. Free Radic. Biol. Med. 1999;26:1405–1417. doi: 10.1016/s0891-5849(98)00337-2. [DOI] [PubMed] [Google Scholar]
- 38.Zhang H. Agardh C.D. Agardh E. Increased catalase levels and hypoxanthine-enhanced nitro-blue tetrazolium staining in rat retina after ischemia followed by recirculation. Curr. Eye Res. 1995;14:47–54. doi: 10.3109/02713689508999913. [DOI] [PubMed] [Google Scholar]
- 39.Chida M. Suzuki K. Nakanishi-Ueda T. Ueda T. Yasuhara H. Koide R. Armstrong D. In vitro testing of antioxidants and biochemical end-points in bovine retinal tissue. Ophthalmic Res. 1999;31:407–415. doi: 10.1159/000055565. [DOI] [PubMed] [Google Scholar]
- 40.Nayak M.S. Kita M. Marmor M.F. Protection of rabbit retina from ischemic injury by superoxide dismutase and catalase. Invest. Ophthalmol. Vis. Sci. 1993;34:2018–2022. [PubMed] [Google Scholar]
- 41.Song Y. Gong Y.Y. Xie Z.G. Li C.H. Gu Q. Wu X.W. Edaravone (MCI-186), a free radical scavenger, attenuates retinal ischemia/reperfusion injury in rats. Acta Pharmacol. Sin. 2008;29:823–828. doi: 10.1111/j.1745-7254.2008.00822.x. [DOI] [PubMed] [Google Scholar]
- 42.Takase S. Lerond L. Bergan J.J. Schmid-Schonbein G.W. The inflammatory reaction during venous hypertension in the rat. Microcirculation. 2000;7:41–52. [PubMed] [Google Scholar]
- 43.Korthuis R.J. Gute D.C. Adhesion molecule expression in postischemic microvascular dysfunction: activity of a micronized purified flavonoid fraction. J. Vasc. Res. 1999;36(Suppl 1):15–23. doi: 10.1159/000054070. [DOI] [PubMed] [Google Scholar]