Figure 3. KCC activity regulated the NADPH oxidase of human neutrophil.
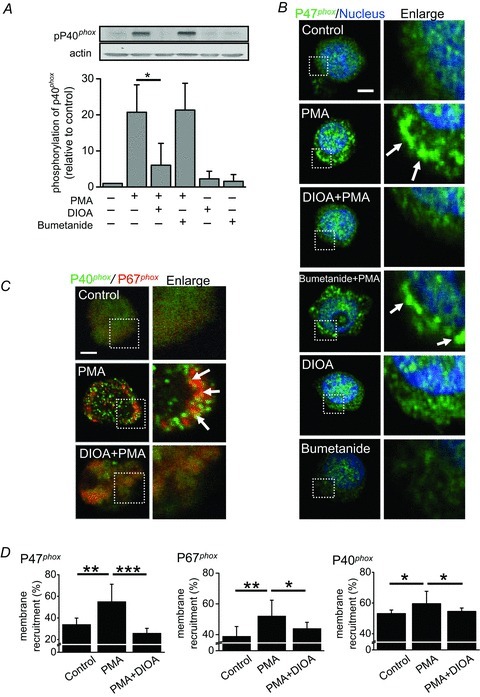
A, the phosphorylation of phox units in human neutrophils. A representative immunoblot showed the phosphorylation levels of p40phox in human neutrophils at various experimental conditions. The control group was neutrophils treated with 0.1% DMSO alone. The detailed protocols for neutrophil stimulation were described in Methods. B and C, PMA induced the membrane recruitments of phox units (p47phox, p40phox and p67phox). PMA caused the puncta formation of p47phox, especially at the membrane periphery (B). DIOA, but not bumetanide, reduced the PMA-provoked membrane recruitment of p47phox proteins. Other oxidase components such as p40phox and p67phox also remained diffusely distributed in cytoplasm during latency. PMA induced the assembly of p40phox and p67phox into complexes that were recruited to the juxta-membrane region (C). D, the quantitative analyses for the membrane recruitment of different phox units at various experimental conditions. Each value represents the mean ± SEM from three different experiments by analysing at least 30 cells. *P≤ 0.05, **P≤ 0.01, ***P≤ 0.005. Scale bar, 2 μm.
