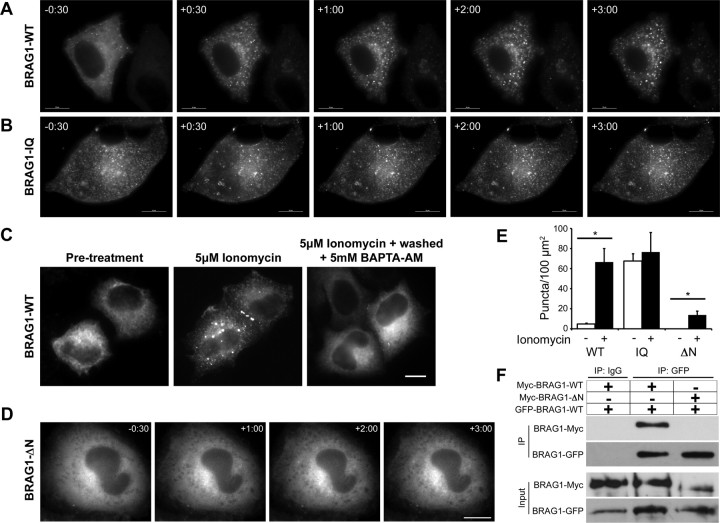
Figure 3.
Ca2+ influx triggers a reversible conformational change in BRAG1. A, B, Formation of cytoplasmic puncta in response to calcium influx. HeLa cells expressing mCherry-BRAG1-WT (A) or mCherry-BRAG1-IQ (B) were stimulated with 5 μm ionomycin for 3 min. Single frames from time-lapse movies beginning at 30 s before ionomycin treatment (−30) are shown. Note that BRAG1-IQ is punctate in the absence of ionomycin and does not change its distribution after ionomycin addition. C, Calcium-induced condensation of BRAG1 is reversible. HeLa cells were either left untreated (left), were treated with 5 μm ionomycin for 3 min (center), or were treated with 5 μm ionomycin for 3 min followed by incubation with 5 mm BAPTA-AM for 10 min (right). D, BRAG1-ΔN does not form puncta in response to ionomycin stimulation. Cells expressing mCherry-BRAG1-ΔN were treated with ionomycin for 3 min and imaged as described above. E, Quantification of calcium-induced puncta formation. Cells expressing BRAG1-WT, BRAG1-IQ or BRAG1-ΔN were treated with either vehicle (DMSO) or ionomycin for 3 min, then fixed and imaged. Puncta were quantified as described previously (see Materials and Methods) from at least 18 cells from three independent experiments. Mean number of BRAG1 puncta per 100 μm2 pre- and post-ionomycin stimulation (WT-pre: 4.6 ± 0.9 puncta, n = 19; WT-post: 66.6 ± 13.5 puncta; n = 19, p < 0.001; IQ-pre: 67.5 ± 7.4 puncta, n = 17; IQ-post: 76.6 ± 19.5 puncta; n = 17, p = 0.25; ΔN-pre: 0.0 ± 0. puncta, n = 18; ΔN-post: 13.8 ± 3.8 puncta; n = 19, p < 0.005). *p ≤ 0.005 (Student's t test). F, The N terminus of BRAG1 is necessary for self-association. Myc-BRAG1-WT or myc-BRAG1-ΔN were coexpressed in HeLa cells with GFP-BRAG1 WT. Cell lysates were then immunoprecipitated with anti-GFP, and the presence of bound myc-BRAG1 detected by immunoblotting with anti-myc. Scale bars: 10 μm.