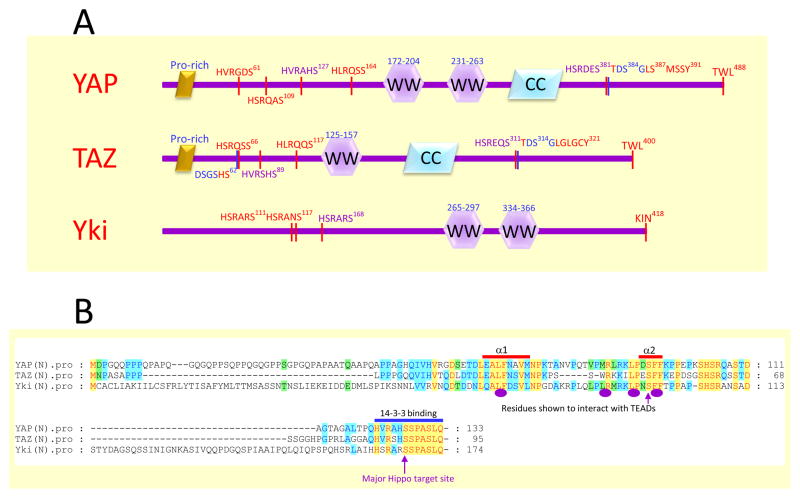
Figure 1.
Domain organization of YAP, TAZ and Yki.
A. The schematic drawing of YAP, TAZ and Yki. The key features are indicated such as the Pro-rich region, the WW domain, and coiled coil region. The HXRXXS motifs targeted by the Hippo pathway are also indicated with the major site in purple. The DSG-degron at the C-terminal part of YAP and TAZ is shown in blue, and the additional N-terminal DSGXXS degron of TAZ is also indicated.
B. Alignment of the N-terminal region of YAP, TAZ and Yki. Residues identical in all three proteins are indicated in red with yellow background. The residues experimentally demonstrated to be involved in interaction with TEADs are indicated with purple ovals (using TAZ in the study) and arrow (using YAP in the analysis) below the alignment. These residues are located within the alpha1 and alpha2 helices (indicated above the alignment), which were revealed structurally to contact TEADs directly. The major Hippo target site whose phosphorylation created a binding site for 14–3–3 proteins is also highlighted.