Figure 3.
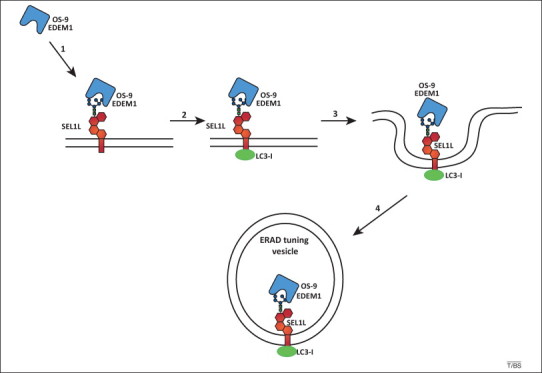
Endoplasmic reticulum-associated degradation (ERAD) tuning model. The receptor-mediated removal of ER-degradation enhancing mannosidase-like protein (EDEM1) and OS-9 from the ER lumen is shown. In the absence of misfolded proteins, type I membrane glycoprotein (SEL1L) is disengaged from the HRD1 dislocation machinery. The penta-glycosylated ectodomain of SEL1L associates with EDEM1 and OS-9 (step 1). The cytosolic tail of SEL1L or a SEL1L-associated protein binds the cytosolic ubiquitin-like protein LC3-I. The complex is released from the ER in vesicles (steps 3 and 4) that may eventually fuse with an ill-defined degradative compartment. ERAD tuning vesicles are co-opted by coronaviruses as replication and transcription platform vesicles.
