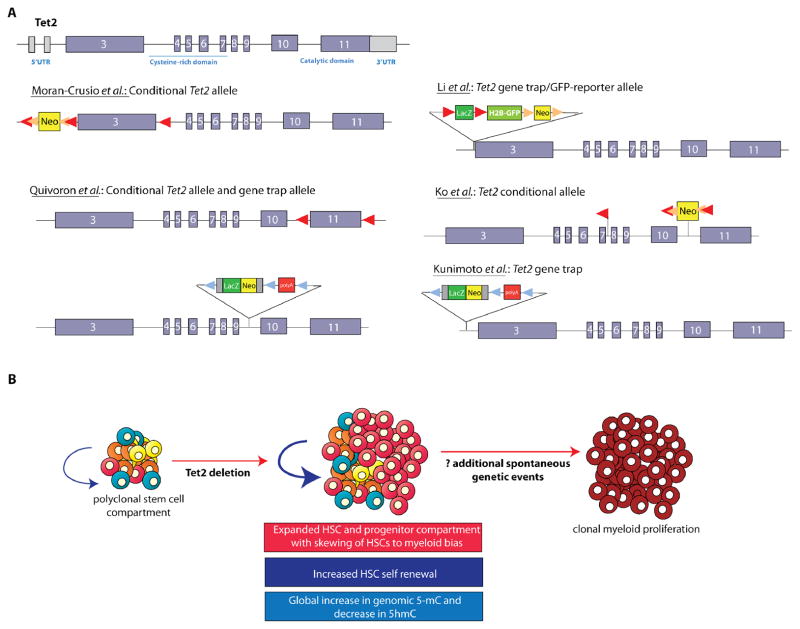
Figure 2. The role of TET2 in hematopoiesis as identified by deletion of Tet2 in murine models.
Targeted disruption of murine Tet2 has now been accomplished using at least six different constructs each targeting Tet2 in a different location and manner (A). This includes conditional deletion of Tet2 by targeting the first coding exon (as done by Moran-Crusio et al.), the last coding exon encoding the enzymatic function of Tet2 (as done by Quivoron et al.), or the exons 8–10 (as done by Ko et al.). Several groups have also studied mice with germline deletion of Tet2 as accomplished by several different gene trap constructs (A). Nearly all of these models have revealed that deletion of Tet2, whether in a conditional, hematopoietic-specific manner, or in the germline, results in expansion of the hematopoietic-stem progenitor compartment and increased hematopoietic stem cell self-renewal shortly after deletion (B). Overt myeloproliferation is also evident following Tet2 deletion in vivo but with a latency of at least 3–6 months, suggesting the acquisition of additional collaborating events are required for disease initiation and progression in vivo.