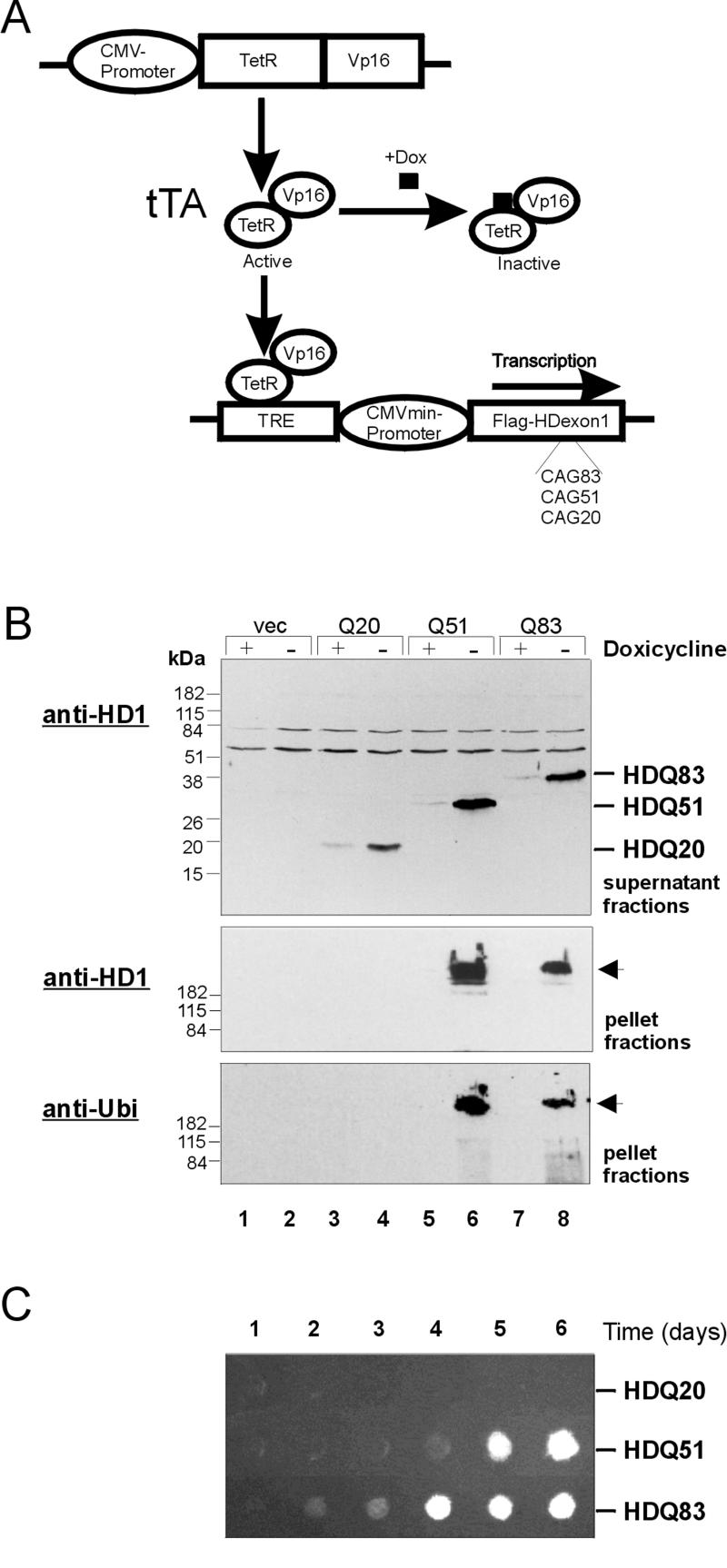
Figure 1.
Cell model design and analysis of HD exon 1 protein aggregation in 293 Tet-Off cells. (A) Activation of the transactivator tTA, a fusion protein consisting of the tet-repressor and the VP16 activation domain, by removal of doxycycline (Dox) from the culture medium results in the binding of tTA to the tet-responsive element TRE and transcription of Flag-tagged HD exon 1 fragments with 20, 51, and 83 CAG repeats in 293 Tet-Off cells. (B) Immunoblot analysis of soluble (supernatant) and insoluble (pellet) protein fractions prepared from induced and uninduced 293 Tet-Off cells. For induction of HD exon 1 expression, cells were grown for 3 d in the absence of doxycycline. Proteins were resolved by SDS-PAGE, blot ted onto nitrocellulose membranes, and probed with the anti-huntingtin antibody HD1 (top and middle panel) or with anti-ubiquitin antibody (bottom panel). Lanes 1 and 2: protein fractions prepared from induced and uninduced control cells lacking the HD exon 1 construct; lanes 3, 5, and 7: protein fractions prepared from uninduced cells; lanes 4, 6, and 8: protein fractions prepared from induced cells expressing the proteins HDQ20, HDQ51, and HDQ83, respectively. Arrows mark the origin of electrophoresis. (C) Time course of HD exon 1 aggregation in 293 Tet-Off cells. Expression of the recombinant proteins was induced by removal of doxycycline from the culture medium. Protein extracts were prepared at the indicated times after induction and analyzed by a filter retardation assay. Captured HD exon 1 protein aggregates were detected with the HD1 antibody.