Abstract
Currently approved combination regimens available for the treatment of metastatic tumors, such as breast cancer, have been shown to increase response rates, often at the cost of a substantial increase in toxicity. An ideal combination strategy may consist of agents with different mechanisms of action leading to complementary antitumor activities and safety profiles. In the present study, we investigated the effects of the epigenetic modulator apicidin in combination with the cytotoxic agent docetaxel in tumor breast cell lines characterized by different grades of invasiveness. We report that combined treatment of apicidin and docetaxel, at low toxicity doses, stimulates in metastatic breast cancer cells the expression of CTCF-like protein and other cancer antigens, thus potentially favoring an antitumor immune response. In addition, apicidin and docetaxel co-treatment specifically stimulates apoptosis, characterized by an increased Bax/Bcl-2 ratio and caspase-8 activation. Importantly, following combined exposure to these agents, metastatic cells were also found to induce signals of immunogenic apoptosis such as cell surface expression of calreticulin and release of considerable amounts of high-mobility group box 1 protein, thus potentially promoting the translation of induced cell death into antitumor immune response. Altogether, our results indicate that the combined use of apicidin and docetaxel, at a low toxicity profile, may represent a potential innovative strategy able to activate complementary antitumor pathways in metastatic breast cancer cells, associated with a potential control of metastatic growth and possible induction of antitumor immunity.
Introduction
Combining therapeutics with strengthened antitumor activity is a major challenge in the clinical management of patients with cancer. The highest therapeutic value of these strategies relies both on ensuring tumor cell death and increasing the immunologic recognition of poorly immunogenic cancer cells [1].
Metastatic breast cancer is a heterogeneous disease characterized by deregulation of multiple cellular pathways governing tumor cell proliferation, survival, cell cycle control, apoptosis, angiogenesis, and metastatic invasiveness [2]. The choice of treatment for this cancer is based on patient-specific factors, such as the expression of estrogen receptor, progesterone receptor, and human epidermal growth factor receptor 2 [3]. Up to now, chemotherapy is the first-line treatment option for the highly metastatic endocrine-resistant cancer types, often associated with substantial toxicity. Advances in therapeutic strategies against breast cancer suggest that combination of selected regimens may produce synergistic efficacy with a manageable toxicity profile. An ideal combination regimen would consist of agents with different mechanisms of action leading to complementary antitumor activities and no cross resistance [4].
The taxane docetaxel is well known for its efficacy in patients with breast cancer but also for its adverse toxic effects due to the dosage [5]. Histone deacetylase inhibitors (HDACi) are a class of anticancer agents with a potential role in the treatment of breast cancer [6]. Among HDACi, apicidin suppresses the growth of human breast cancer cells by modulating cell cycle and inducing apoptosis [7–9]. Apicidin in combination with doxorubicin significantly increases apoptosis and caspase activation in hepatocellular carcinoma leading to significantly amplified antitumor effects compared to the treatment with either apicidin or doxorubicin alone [10]. Interestingly, apicidin was selected as a suitable starting point for developing a second generation of HDACi with better potency [11]. In the light of this, it is of great interest to investigate the antitumor activity as well as the adverse effects exerted by a combination regimen with apicidin and docetaxel for treating metastatic breast cancer.
One of the potential immunomodulatory effects of a combined therapy based on the use of apicidin and docetaxel is the activation of cancer testis (CT) genes, the most immunogenic cancer antigens known to date [12]. Among these, CTCF-like protein (CTCFL), also called Brother of the Regulator of Imprinted Sites, has been found to be deregulated in various types of tumor and to control the expression of the well-known tumor-associated antigens NY-ESO-1 and MAGE-A1 [13–15]. CTCFL is overexpressed by several breast cancer cell lines and by about 70% of human breast tumor tissues [16]. Of interest, it has also been detected in leukocytes of patients with breast cancer as an early marker of tumorigenesis [17]. Notably, CTCFL tested in the setting of an anticancer vaccine for the treatment of the highly metastatic and poorly immunogenic 4T1 mammary carcinoma elicited a strong immune response able to delay the appearance of tumors and to prolong significantly survival of the mice [18].
Overcoming apoptosis resistance of cancer cells remains one of the major objectives of new potential strategies of treatment for breast cancer, including combination therapies [19]. BCL-2 family members include important regulators of apoptosis whose modulated expression in breast cancer cells is tightly associated with the response to therapeutic treatments as well as to disease outcome [20]. Therefore, regulators such as the antiapoptotic Bcl-2 and the proapoptotic Bax remain major targets of anticancer therapies [21]. Recently, the concept of immunogenic apoptosis has been associated with a chemotherapeutic agent-specific cancer cell death modality able to potentiate anticancer immunity similarly to an anticancer vaccine [22]. Immunogenic apoptosis occurs through activation of diverse cellular signals, including the translocation of calreticulin (CRT) from inside the cell to the surface as well as the release of high-mobility group box 1 protein (HMGB1). CRT is supposed to act not only as an important “eat me” signal for professional phagocytes but also as a central anticancer immunity mediator, thus playing an active role in inducing antitumor immune response [23]. Moreover, another important inflammatory signal related to immunogenic apoptosis is given by HMGB1, a chromatin-associated nuclear protein subjected to a large number of post-transcriptional modifications regulating its trafficking from the nucleus to the cytoplasm and its further release. This protein is released in the extracellular compartment by apoptotic cells during the late phase of cell death and acts as a proinflammatory signal able to trigger a potent immune response [24]. This event may also occur throughout the activation of dendritic cells (DCs) that increase antigen processing and presentation, thus determining efficient cytotoxic T-cell activation [25].
In the present study, we investigated the antitumor efficacy of a new therapeutic regimen based on the combination of the epigenetic compound apicidin and the cytotoxic agent docetaxel in three different breast cancer cell lines characterized by diverse grade of tumorigenicity and metastatic behavior. We evaluated the molecular events potentially leading to the effectiveness of this therapeutic option in metastatic breast cancer cell lines and found the occurrence of direct as well as immune-mediated antitumor signals. Co-treatment of apicidin and docetaxel, at a low toxicity profile, induced high expression of CTCFL in highly metastatic breast cancer cells. Moreover, these cells exposed to apicidin and docetaxel combined treatment exhibited increased apoptosis associated to Bcl-2 down-modulation. Importantly, cell death induced by this combined treatment showed signals of immunogenic apoptosis such as CRT exposure on cell surface and HMGB1 release. On the whole, these events may ensure at once induction of immunomodulatory events and tumor cell death, possibly enhancing the antitumor response toward poorly immunogenic breast cancer cells.
Materials and Methods
Cell Culture
The following three breast cell lines with different tumorigenicity/invasiveness ability were used: highly metastatic MDA-MB-435 cells, invasively growing MDA-MB-231 cells, and poorly invasive MCF-7 cells [26]. Cell cultures were maintained at 37°C, with 5% CO2 under fully humidified conditions in RPMI-1640 (Lonza, Verviers, Belgium) supplemented with 10% FBS (EuroClone, West York, United Kingdom), 2 mM glutamine, 100 U/ml penicillin, and 100 µg/ml streptomycin (Lonza).
Drug Treatment Regimens
Apicidin was purchased from Sigma Inc. (St Louis, MO). Docetaxel was kindly provided by G. Pascale Institute (Naples, Italy).
Cell Viability Assay
CellTiter 96 AQueous One Solution Cell Proliferation Assay (Promega, Madison, WI) was used to determine cytotoxicity effects after treatment with drugs. Absorbance at 450 nm was determined on Opsys MR spectrophotometer (DYNEX Technologies, Denkendorf, Germany), using Windows Revelation QuickLink software. Cells were plated at a density of 5 x 103 cell/well in 96-well plates, and then treated with various concentrations of apicidin and docetaxel for 24, 48, and 72 hours. At the end of treatment period, 20 µl of 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium (MTS) reagent were added to each well. The plates were then incubated for 2 hours at 37°C in the dark. Each experimental condition was performed in triplicate and repeated at least twice. All values were normalized with respect to the viability of untreated cells.
RNA Isolation, Reverse Transcription, and Quantitative Real-time Polymerase Chain Reaction Analysis
Total RNA was isolated from cultured cells with RNeasy Mini Kit (Qiagen, Chatsworth, CA), according to the manufacturer's instructions, quantified using Thermo Scientific NanoDrop ND-100 (Wilmington, DE), and then converted to cDNA using random primers and ThermoScript reverse transcriptase (Invitrogen, Carlsbad, CA). Quantitative real-time polymerase chain reaction (qPCR) analysis was performed using the Applied Biosystems 7500HT Real-Time PCR System. CTCFL expression was determined by using the following primers/probe sequences: 5′-CCCATTGTGCCACCATCA-3′ (sense), 5′-AGCATGCAAGTTGCGCATAT-3′ (antisense), and 6FAM-ACGGAAAAGCGACCTAC-MGB (CTCFL probe) [15]. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) primers/probe mixture was determined by Predeveloped Assay (Applied Biosystems, Foster City, CA). NY-ESO-1 and MAGE-A1 expression was analyzed by using the following primer sequences: 5′-CCCCACCGCTTCCCGTG-3′ (NY-ESO-1 forward), 5′-CTGGCCACTCGTGCTGGGA-3′ (NY-ESO-1 reverse), 5′-CGGCCGAAGGAACCTGACCCAG-3′ (MAGE-A1 forward), and 5′-GCTGGAACCCTCACTGGGTTGCC-3′ (MAGE-A1 reverse). PCR reactions were prepared using 2.0 µl of cDNA diluted in SensiMix SYBR Kit (Bioline, London, United Kingdom). Amounts of mRNA transcripts were expressed in relative copy numbers normalized to the housekeeping gene GAPDH (comparative Ct method).
Detection of Cell Death
Apoptosis and necrosis were assessed by flow cytometry following fluorescein isothiocyanate (FITC)-conjugated Annexin V (Annexin V-FITC) and propidium iodide (PI) staining (ApoAlert; Clontech Laboratories, Palo Alto, CA). Briefly, after a 24-hour drug treatment, cells were harvested by trypsinization, pooled, washed twice with PBS, and incubated with Annexin V-FITC and PI, according to the manufacturer's instructions. Cells were analyzed using FACSCalibur (Becton Dickinson, Franklin Lakes, NY). Data analysis was performed by WINMDI software and expressed as percentage of positive cells.
DC Generation and Drug Treatments
Peripheral blood mononuclear cells (PBMCs) were purified from heparinized blood obtained from healthy donors on a density gradient (Lymphoprep; Nycomed Pharma AS, Oslo, Norway). Monocytes were positively sorted using anti-CD14-labeled magnetic beads (MACS; Miltenyi, Bergisch Gladbach, Germany), according to the manufacturer's instructions. Freshly isolated monocytes were resuspended in complete medium with 10% FBS containing granulocyte-macrophage colony-stimulating factor (GM-CSF; 50 ng/ml) and interleukin-4 (IL-4; 1000 U/ml) at 2 x 106 cell/ml in 6-well plates for 5 days. On day 5, DCs were collected, washed, and cultured in the presence or absence of the drugs for 24 hours. Immunofluorescent staining was performed to detect human leukocyte antigens (HLA-DR), CD83, CD80, CD86, and CD40 molecules (BD Biosciences).
Western Blot Analysis
CTCFL expression was assessed by lysing cells with a high salt lysis buffer containing 500 nM Tris-HCl (pH 7.5), 300 nM NaCl, 1% NP40, 5 mM EDTA, and a protease inhibitor mixture (Roche Diagnostics, Mannheim, Germany). Equal amounts of total proteins were boiled in sample buffer and separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). Following immunoblot analysis with a polyclonal rabbit antibody (Ab) against CTCFL (Abcam, Cambridge, United Kingdom), immunoreactive bands were visualized by using HRP-conjugated secondary Ab and the ECL system (Amersham Biosciences, Buckingham, United Kingdom). To examine major histocompatibility complex class I (MHC I), Bcl-2, Bax, and caspase-8 expression, we extracted cells with a modified RIPA lysis buffer [250 mM Tris-HCl (pH 8), 137 mM NaCl, 2 mM EDTA (pH 8), 0.5% NP40, 10% glycerol, and protease inhibitor mixture (Roche Diagnostics)]. Equal amounts of total proteins were boiled in sample buffer and separated by SDS-PAGE. Following alternative immunoblot analysis with Abs [mouse anti-human HLA-ABC, mouse anti-human Bcl-2 (Dako, Glostrup, Denmark); rabbit anti-human Bax (Santa Cruz Biotechnology, Santa Cruz, CA); mouse anti-human caspase-8 (Cell Signaling Technology, Danvers, MA)], immunoreactive bands were visualized by using the specific HRP-conjugated secondary Ab and the ECL system (Amersham Biosciences). HMGB1 expression was analyzed in cell supernatants. Briefly, equal amounts of each sample normalized by cell number were separated by SDS-PAGE, and immunoblot analysis was performed with a rabbit polyclonal Ab to HMGB1 (Abcam). The optical density of the bands [integrated area in arbitrary units (AU)] was measured by using the National Institutes of Health Image J software (rsb.info.nih.gov/ij).
Confocal Laser Scanning Microscopy Analysis
For confocal laser scanning microscopy (CLSM) analysis, cancer cell lines were seeded in 24-well cluster plates on cover glasses (diameter: 12 mm; 2 x 105 cells/well). After a 48-hour drug treatment, cells were fixed by 3% paraformaldehyde (30 minutes at 4°C), permeabilized by 0.5% Triton X-100 (10 minutes at room temperature; Sigma-Aldrich), and then stained at 37°C with rabbit polyclonal anti-CTCFL or anti-HMGB1 Abs (Abcam) followed by Alexa Fluor 488-conjugated secondary Abs (Molecular Probes, Eugene, OR). The specificity of the polyclonal anti-CTCFL Ab was tested by staining cells with Ab preincubated with the immunogenic peptide, as negative control. Afterward, the cover glasses were extensively washed with PBS and then mounted on the microscope slide with Vectashield antifade mounting medium (Vector Laboratories, Burlingame, CA) containing 4,6-diaminidino-2-phenylindole (DAPI). CLSM observations were performed with a Leica TCS SP-2 AOBS apparatus, using a 63x/1.40 NA oil objective and 405- to 488-nm excitation spectral laser lines appropriately tuned by acousto-optical tunable filter. Image acquisition and processing were carried out using the Leica Confocal Software 2.6 rel 1537 (Leica, Lasertechnik, Heidelberg, Germany) and Adobe Photoshop CS2 1.4.10 software programs (Adobe Systems, San Jose, CA). Signals from different fluorescent probes were taken in sequential scan settings. Different fields of view were analyzed on the microscope for each labeling condition, and representative results are shown.
Membrane CRT Expression
Immunofluorescent staining was used to detect CRT exposure in breast cancer cell lines treated with drugs. Briefly, 2 x 105 cells were plated in 6-well plates and, 24 hours after treatment, were collected with EDTA, washed twice with PBS, and incubated for 30 minutes with a goat CRT-specific polyclonal Ab diluted 1:10 (Santa Cruz Biotechnology). After a 10-minute incubation with cold blocking buffer (human AB serum), cells were washed and incubated with the FITC-conjugated polyclonal secondary Ab diluted 1:500 (30 minutes). Samples were analyzed by FACSCalibur (Becton Dickinson) and WINMDI software. Isotype-matched IgG Abs were used as control.
Statistical Analysis
Data were represented as means ± SD of determinations from either three or more independent experiments. Statistical analysis was carried out using unpaired Student's t test. Values were considered as significant when the probability was below the 5% confidence level (P ≤ .05).
Results
Combined Treatment with Apicidin and Docetaxel Strongly Modulates CTCFL in Highly Metastatic Breast Cancer Cells and Exerts Immunomodulatory Effects
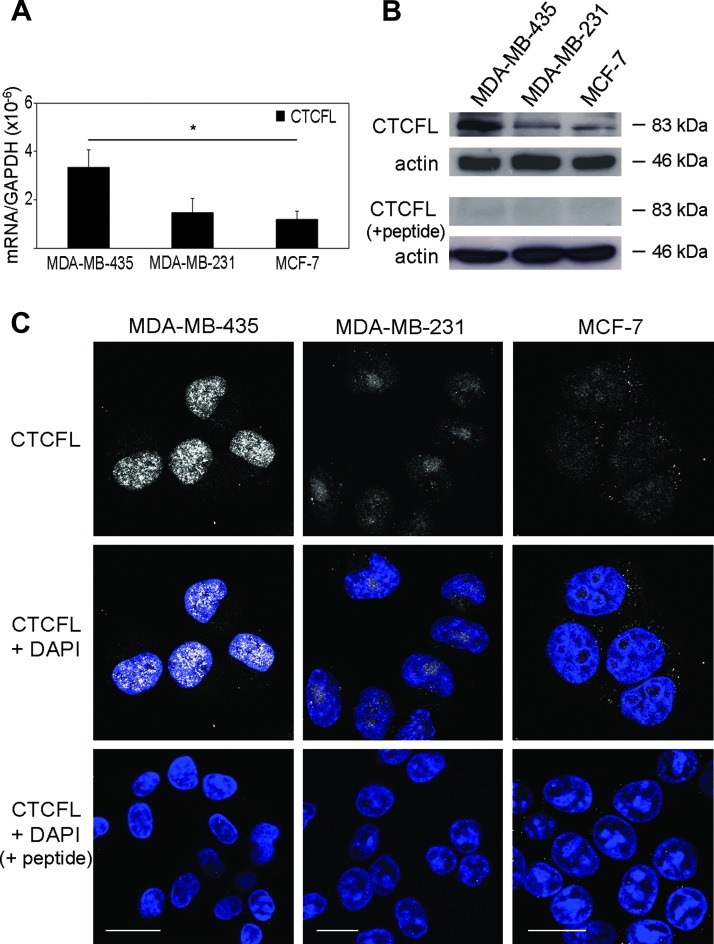
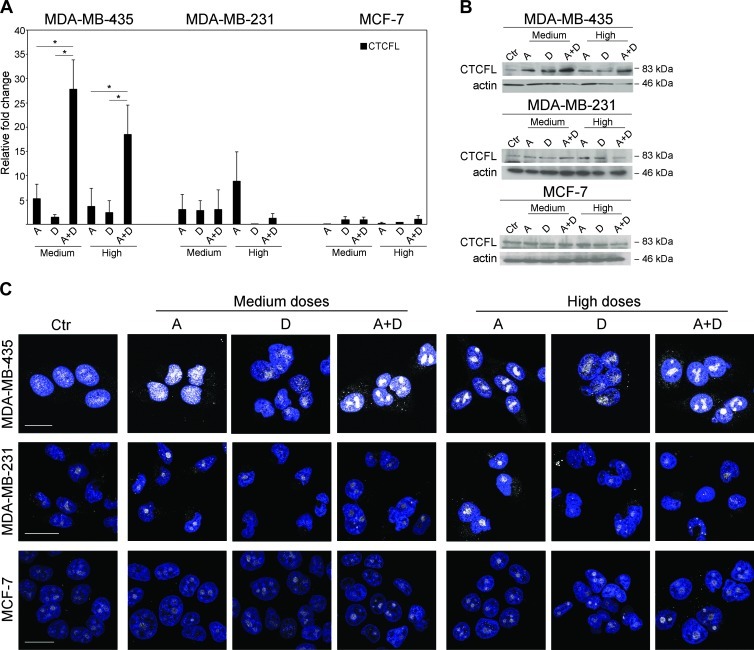
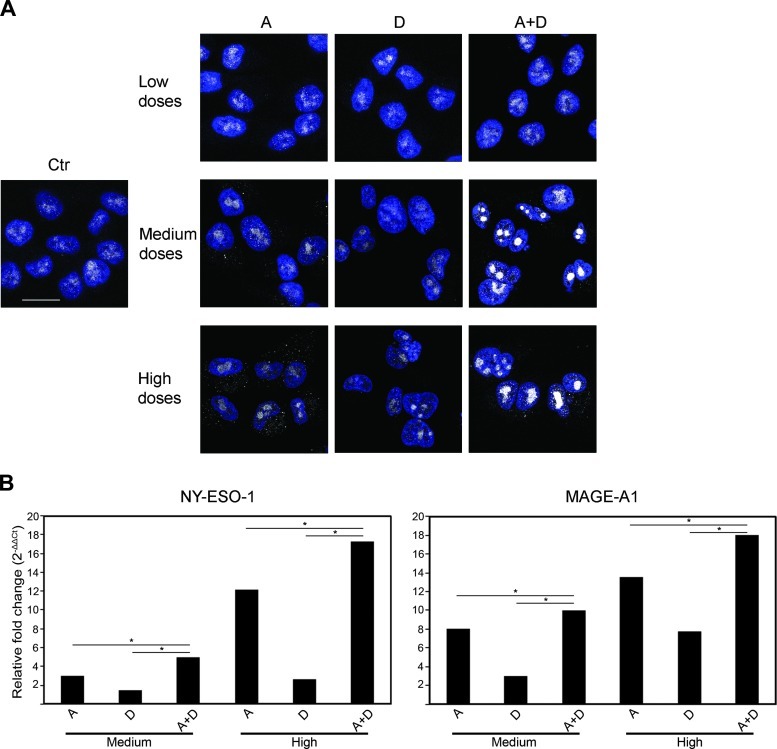
Apicidin has been shown to suppress the growth of human breast cancer cells, whereas docetaxel is one of the most effective chemotherapeutic agent with antiproliferative effect on breast cells [7,27]. The potentially immunogenic CTCFL is absent in normal breast cells, whereas it is present at variable levels in all breast cancer cell lines, both in the nucleus and in the cytoplasm [16]. Thus, we investigated whether the co-treatment with apicidin and docetaxel stimulated the immunogenicity of breast cancer cells by inducing high expression of CTCFL at low toxicity doses. To this end, we first examined CTCFL basal expression in three breast cancer-derived cell lines with different grades of tumorigenicity, i.e., the highly metastatic MDA-MB-435, the invasively growing MDA-MB-231, and the poorly invasive MCF-7 cells. CTCFL mRNA and protein, assayed by qPCR, Western blot, and CLSM analyses, were detected at high levels in MDA-MB-435 cells but barely in MDA-MB-231 and MCF-7 cells (Figure 1). No staining was detected in the presence of CTCFL-specific peptide blocking CTCFL Ab in all tested breast cancer cell lines (Figure 1, B and C). In agreement with earlier reports, these data confirmed that CTCFL is present at variable levels in breast cancer cell lines with different grades of tumorigenicity [16,28]. Then, to choose the optimal dosage of drugs with a low toxicity profile able to induce CTCFL, we tested apicidin and docetaxel to cause cytotoxic effects on breast cancer cell lines in vitro by using MTS assay. Thus, cells were treated with rising doses of drugs for 24, 48, and 72 hours. We found that both agents inhibited cell proliferation in a time- and dose-dependent manner (Figure W1). Rising doses of apicidin determined growth inhibition and cell death at the same extent in all breast cancer cell lines, whereas docetaxel exhibited a stronger antiproliferative effect against MDA-MB-435 and MDA-MB-231 cells rather than MCF-7 cells. These results defined the nontoxic doses of apicidin and docetaxel, closest to the IC20 values for all tested cell lines at 48-hour treatment, as the highest nontoxic concentrations to be used in our experiments. This allowed us to preserve cell viability while studying the mechanism of action of these drugs. Thus, the experiments were performed by using 100, 500, and 1000 nM apicidin and 0.1, 1, and 2.5 nM docetaxel, namely, as low, medium, and high doses, respectively. We then assessed the modulation of CTCFL in all tested breast cancer cell lines following co-treatment with apicidin and docetaxel. Combined administration of medium doses, and to a lesser extent of high doses, of these drugs resulted in a marked increase of CTCFL expression, both at mRNA and protein levels, in the highly metastatic MDA-MB-435 cells compared to single drug treatments (Figure 2, A and B). In MDA-MB-231 cells, CTCFL modulation occurred under high-dose apicidin treatment (Figure 2, A and B). By contrast, the same treatments, at all tested concentrations, did not induce any CTCFL modulation in MCF-7 cells (Figure 2, A and B). We further investigated the intracellular localization of CTCFL after co-treatment with apicidin and docetaxel by CLS Manalysis. We found that, although apicidin alone induced a moderate increase of CTCFL expression in MDA-MB-435 cells, combined treatment with medium doses of apicidin and docetaxel for 48 hours caused a clear-cut up-regulation of this protein, which accumulated in defined nuclear areas faintly stained with DAPI, indicating euchromatin regions. Consistent with qPCR and Western blot analyses, the expression and distribution of CTCFL did not significantly change in MDA-MB-231 and MCF-7 cells in any experimental conditions (Figure 2C). Of interest, combined low doses of drugs determined a significant CTCFL overexpression only in MDA-MB-435 cells, although to a lesser extent than that occurring at medium-dose co-treatment (Figure W2, A and B). To specifically evaluate the persistence of CTCFL overexpression, we performed CLSM analysis in MDA-MB-435 cells subjected to long-term drug co-treatment. We found that after a 72-hour exposure to low doses of combined drugs the expression of CTCFL returned at basal levels, whereas medium and high doses of drugs prolonged the persistence of CTCFL expression, thus suggesting its transient control induced by the combined treatment with apicidin and docetaxel in a dose- and time-dependent manner (Figure 3A). Given the selectively nuclear accumulation of CTCFL following combined treatment with apicidin and docetaxel at certain doses and its persistence overtime, we sought to assess whether the modulation of MAGE-A1 and NYESO-1, reported to be CTCFL targets, occurred in treated MDAMB-435 cells. qPCR analysis revealed significant up-modulation of NY-ESO-1 and, to a lesser extent, MAGE-A1 after a 48-hour treatment of cells with high doses of combined drugs (Figure 3B). Conversely, MDA-MB-231 and MCF-7 cells did not display any significant regulation of both genes under the same experimental conditions (data not shown). Of interest, we also investigated the expression of MHC I on cancer cells as sign of an efficient presentation of tumor antigens to CD8+ T lymphocytes [29], and we found that combined high doses of apicidin and docetaxel, over the single treatments, increased the expression of this molecule significantly in the highly metastatic MDA-MB-435 cells (Figure W3).
Figure 1.
CTCFL is differently expressed in MDA-MB-435, MDA-MB-231, and MCF-7 cell lines. (A) qPCR analysis of CTCFL transcripts in selected human breast cell lines. Levels of CTCFL mRNA were calculated using absolute normalization of Ct and normalized to GAPDH levels (ΔCt). Experiments were performed in triplicate, and each value represents mean ± SD of three independent experiments, *P < .05. (B) One representative Western blot analysis of three of total cell lysates showing the total content of CTCFL in the different breast cancer cell lines. Actin was used as quantitative loading control. In the lower panel, the blot was incubated with CTCFL-specific peptide blocking CTCFL Ab, as negative control. (C) CLSM analysis of fixed and permeabilized cells stained for intracellular CTCFL (gray). Offset and gain values of the photomultiplier channel were regulated with respect to the setup selected for MDA-MB-435 cell analysis to make fluorescence intensity comparable across experiments on different samples. Nuclei were stained with DAPI (blue). The specificity of the polyclonal Ab was assessed by staining cells with a combination of anti-CTCFL Ab and the immunogenic peptide. A representative example of four independently repeated experiments is shown. Scale bars, 20 µm.
Figure 2.
Combined treatment of apicidin and docetaxel induces CTCFL expression in metastatic breast cancer cells. (A) CTCFL mRNA was measured by qPCR in all breast cancer cell lines after 24-hour treatments, and the relative levels were calculated using comparative Ct method (ΔΔCt) and normalized to GAPDH levels. Data represent the mean relative fold changes ± SD of three independent experiments, *P < .05. (B) CTCFL Western blot analysis of cell lysates untreated or after 48-hour treatment with medium and high doses of A and D, alone or in combination. (C) Intracellular CTCFL expression monitored by CLSM on cells exposed for 48 hours to different doses of A and D, alone or in combination. Cells, fixed and permeabilized, were stained with polyclonal anti-CTCFL Ab (gray). Nuclei are shown in blue (DAPI). A representative example of three independent experiments is reported. Scale bar, 20 µm. Ctr, untreated; A, apicidin; D, docetaxel, A + D, apicidin and docetaxel combined treatment.
Figure 3.
Combination of apicidin and docetaxel upregulates persistent CTCFL expression and other CTs in MDA-MB-435 cells. (A) CLSM analysis of fixed and permeabilized MDA-MB-435 cells treated with A and D, alone or in combination, for 72 hours; Ab detection of intracellular CTCFL (gray) is shown; nuclei are stained by DAPI in blue. A representative example of three independently repeated experiments is reported. Scale bars, 20 µm. (B) NY-ESO-1 and MAGE-A1 mRNA expression was evaluated by qPCR in the three cell lines after single and combined treatments with medium and high doses of A and D. Expression levels were calculated using the comparative Ct method (ΔΔCt) and normalized to GAPDH expression. A representative example of three independently repeated experiments is shown, *P < .05.
Apicidin and Docetaxel Combined Treatment Induces Activation of DCs
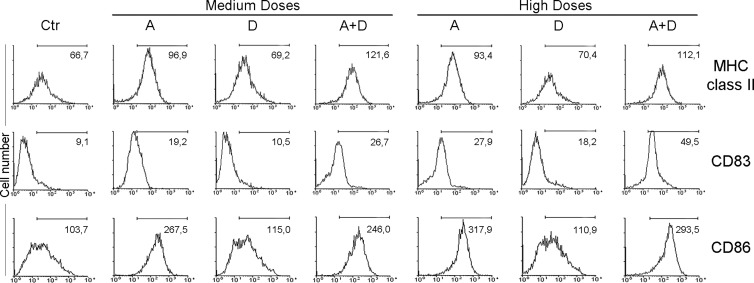
In the light of the strong modulation induced by combined apicidin and docetaxel treatment on CT expression, we next assessed whether apicidin and docetaxel co-treatment could affect the immunostimulatory functions of DCs, major antigen-presenting cells whose activity is known to be potentiated in effective antitumor immune responses generated by drug treatments [30]. Therefore, monocyte-derived DCs were treated with the two drugs, alone or in combination, for 24 hours and analyzed for the expression of maturation and activation markers by fluorescence-activated cell sorting (FACS) analysis. Upon treatment with medium and high doses of apicidin alone, we found a significant increased expression of the maturation and activation molecules MHC II, CD86, and CD83 (Figure 4). Of interest, the combination treatment of apicidin and docetaxel significantly enhanced CD83 expression (Figure 4). By contrast, CD40 or CD80 levels did not change in the same experimental conditions (data not shown). These results demonstrate that apicidin and docetaxel co-treatment is able to elicit DC maturation and activation, thus favoring their functional activity in the tumor microenvironment.
Figure 4.
Apicidin and docetaxel co-treatment modulates activation and maturation markers on DCs. FACS analysis of MHC II, CD83, and CD86 expression on monocyte-derived DCs cultured with A and D, alone or in combination, for 24 hours. The percentage of positive cells is shown for each panel. Similar data were obtained in three independent experiments using three different donors.
Apicidin and Docetaxel Co-treatment Promotes Apoptosis Selectively in Metastatic Breast Cancer Cells
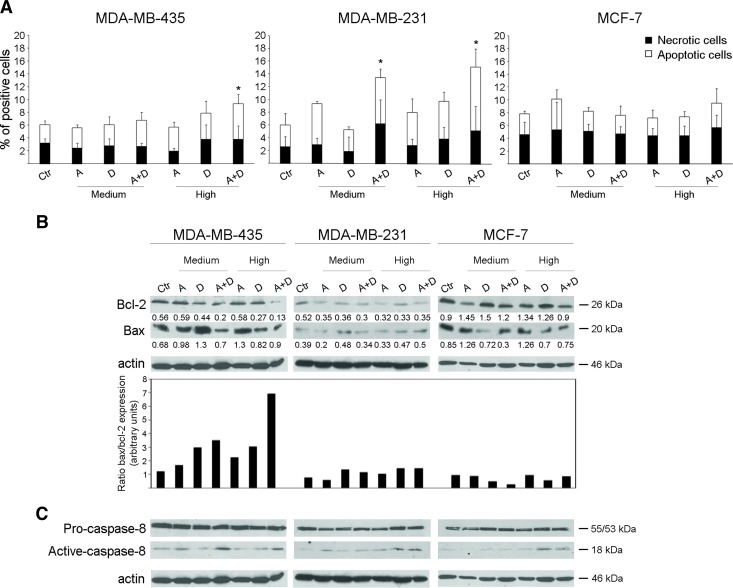
Overcoming resistance to apoptosis remains a major objective of therapeutic treatments in breast cancer [20]. Therefore, we sought to investigate whether apicidin and docetaxel combined treatment exerted an additive effect on inducing apoptosis in metastatic breast cancer cells. First, we treated MDA-MB-435, MDA-MB-231, and MCF-7 cells with medium and high doses of both drugs, alone or combined, for 24 hours and measured apoptosis by Annexin V and PI staining. As shown in Figure 5A, high doses of combined drugs, with respect to the single agents, induced significant apoptosis, revealed by the increased number of Annexin V+/PI- and Annexin V+/PI+ cells, in both MDA-MB-435 and MDA-MB-231 cells. Moreover, this latter cell line showed an increased apoptosis following co-treatment with medium doses of apicidin and docetaxel (Figure 5A). By contrast, no significant additive apoptotic effects were observed in MCF-7 cells at any dose of drugs in combination (Figure 5A). To further dissect the potential proapoptotic effects of apicidin and docetaxel combined treatment, we assessed whether these drugs determined the modulation of two pivotal members of the BCL-2 family, namely, Bcl-2 and Bax, and the activation of caspase-8. We found that both high and medium doses of combined drug treatment of MDA-MB-435 cells for 48 hours significantly reduced the levels of the antiapoptotic protein Bcl-2, whereas the expression of the proapoptotic protein Bax did not change in the same experimental conditions (Figure 5B). These simultaneous events determined a marked enhancement of Bax/Bcl-2 ratio specifically in high drug dose-treated MDA-MB-435 cells (Figure 5B). Of interest, although MDA-MB-231 cells resulted susceptible to apoptosis induced by medium and high combined doses of apicidin and docetaxel, they did modulate neither Bcl-2 expression nor Bax/Bcl-2 ratio, similarly to what was observed in MCF-7 cells (Figure 5B). Concurrently, a significant activation of caspase-8 was also found in MDA-MB-435 cells upon co-treatment with medium and high doses of apicidin and docetaxel with respect to any agent alone, whereas this event was minimal in both MDAMB-231 and MCF-7 cells under the same experimental conditions (Figure 5C).
Figure 5.
Apicidin and docetaxel combined treatment stimulates proapoptotic signals in metastatic breast cancer cells. (A) FACS analysis of apoptosis (Annexin V+PI- and Annexin V+PI+) and necrosis (Annexin V-PI+) in MDA-MB-435, MDA-MB-231, and MCF-7 cell lines treated with medium or high doses of A, D, or A + D for 24 hours. All data are expressed as mean ± SD of five independent experiments. Statistical significance was performed by comparison of each treated cell group with Ctr cells, *P < .05. (B) Bcl-2 and Bax protein detection by Western blot analysis of lysates from 48-hour treated cells with medium and high doses of A and D, alone or in combination. Intensities of both Bcl-2 and Bax bands were measured and values normalized to actin are expressed as AU at the bottom of each panel; Bax/Bcl-2 ratio expression was evaluated on the normalized values and shown in the bottom panel. One representative experiment of three is shown. (C) Pro-caspase-8 and active caspase-8 protein expression of 48-hour treated cells with medium and high doses of A and D, alone or in combination. One experiment of two is shown.
Taken together, these data demonstrate that the combination of high doses, more than medium ones, of apicidin and docetaxel compared to single-agent treatment was effective in stimulating apoptosis signals in the highly metastatic MDA-MB-435 cells and, to a lesser extent, in the invasively growing MDA-MB-231 cells, whereas the nonmetastatic MCF-7 cells were completely refractory to these effects.
Apicidin and Docetaxel in Combination Induce Signals of Immunogenic Apoptosis and HMGB1 Release in Metastatic Breast Cancer Cells
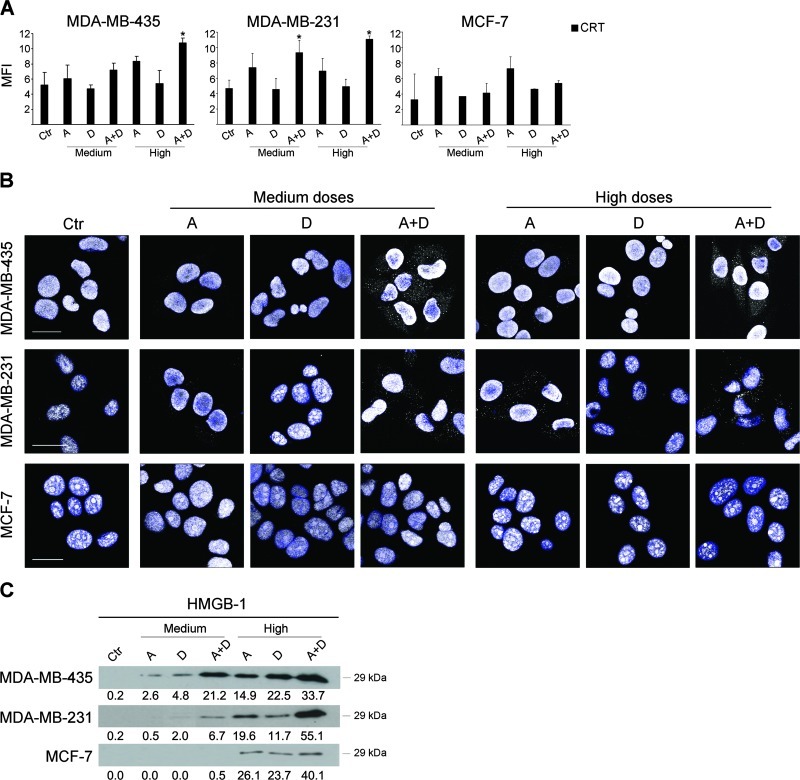
Given the immunomodulatory activities and the proapoptotic effects of combined apicidin and docetaxel in the highly metastatic MDAMB-435 cells, we sought to investigate whether this coupling was effective also in inducing immunogenic apoptosis, recently emerged as chemotherapeutic agent-specific immunogenic cancer cell death modality able to induce “anticancer vaccine effect” [23]. Thus, we assessed two typical signals distinguishing immunogenic apoptosis such as early cell surface exposure of CRT and HMGB1 extracellular release. Of interest, we found that high doses of combined apicidin and docetaxel, over the single treatments, significantly increased the levels of CRT on cell membrane in highly metastatic MDA-MB-435 cells after a 24-hour treatment, whereas the invasively growing MDAMB-231 cells exhibited high levels of CRT on cell surface following treatment with both medium and high doses of combined drugs (Figure 6A). By contrast and in agreement with the apoptosis data, no significant induction of CRT was observed on the surface of MCF-7 cells. Next, the assessment of the expression and localization of HMGB1, deemed as potential immune-response inducer and secreted during late stages of apoptosis, provided the most striking signal of immunogenic apoptosis induced by apicidin and docetaxel combined treatment with respect to any agent alone inmetastatic breast cancer cells. As shown in Figure 6B, a clear-cut accumulation of HMGB1 in cytoplasm occurred specifically in MDA-MB-435 cells and, to a lesser extent, in MDA-MB-231 cells, upon 48-hour co-treatment with medium or high doses of combined apicidin and docetaxel, as determined by CLSM analysis. Of note, this phenomenon was not detectable in the nonmetastatic MCF-7 cells under the same experimental conditions (Figure 6B). Consistent with this, the Western blot analysis of supernatants of tumor cell cultures strikingly confirmed a synergistic or additive effect of the combined treatment with apicidin and docetaxel, respectively, at medium and high doses, in inducing secretion of HMGB1 by MDA-MB-435 cells. Of interest, this phenomenon was much less evident in MDA-MB-231 cells at the medium doses of drug combination (Figure 6C). By contrast, detectable levels of HMGB1 were found in the supernatants of nonmetastatic MCF-7 cells only following treatment with high doses of single or combined drugs (Figure 6C). Notably, the marked synergistic activity of apicidin and docetaxel in inducing increased expression of HMGB1 was not observed upon low doses of combined drug treatment in all breast cancer cell lines (Figure W4). These data demonstrate that apicidin and docetaxel, at a low toxicity profile, act synergistically in inducing signals of immunogenic apoptosis such as cell surface CRT expression and release of extracellular HMGB1 in metastatic breast tumor cells, thus potentially translating induced cell death into an antitumor immune response.
Figure 6.
Apicidin and docetaxel combined treatment induces CRT exposure on cell surface and HMGB1 release in metastatic breast cancer cells. (A) FACS analysis of CRT expression was evaluated as mean fluorescence intensity (MFI) on live breast cancer cell lines (PI- gate). Data are represented as mean value ± SD of three independent experiments. P values were calculated with respect to Ctr, *P < .05. (B) CLSM detection of HMGB1 by a polyclonal rabbit anti-human Ab in MDA-MB-435, MBA-MB-231, and MCF-7 cells exposed for 48 hours to medium and high doses of A and D, alone or in combination (gray, arrows). Nuclei stained with DAPI are blue. Three independent experiments were performed on each cell line. Scale bar, 20 µm. (C) HMGB1 extracellular release assayed by Western blot analysis of supernatants from breast cancer cell lines treated for 48 hours with medium and high doses of A and D, alone or in combination. Intensities of bands were measured and values expressed as AU are given at the bottom of each panel; four independent experiments were performed.
Discussion
New combination therapies for cancer are highly expected to obtain enhanced efficacy and improved selectively along with low toxicity. Indeed, the major objective of drug combination is to keep under control multiple or alternative cellular pathways that result deregulated in cancer cells avoiding drug resistance and subsequent clinical relapse [31]. Enhancing immunogenicity of cancer cells and simultaneously ensuring tumor cell death may potentially improve the response to therapeutic treatment by activation of direct and immune-mediated antitumor responses. On this basis, numerous HDACi in conjunction with other treatments such as chemotherapy, biologic therapy, or radiation therapy are currently under investigation in clinical trials [32,33]. A phase II study of the HDACi vorinostat combined with tamoxifen for the treatment of patients with hormone therapy-resistant breast cancer has shown that this therapeutic combination treatment exhibits encouraging activity in reversing hormone resistance [34]. Similarly, other studies suggest that manipulating protein acetylation by HDACi in combination with hormonal therapies is a promising approach in breast cancer [35]. Very recently, two reports have highlighted that HDACi may potentiate anticancer effect of docetaxel in hormone refractory prostate cancer cells through modulation of Bcl-2 family proteins [36,37]. In this study, we report that the combination of low doses of the HDACi apicidin and the taxane docetaxel, acting through complementary molecular mechanisms, provides additive anticancer effects over single-agent treatment with low toxicity profiles in metastatic breast cancer cells. Our results show that, in the highly metastatic MDA-MB-435 cells, the combination of these agents, with respect to the single treatments, causes the simultaneous activation of diverse cellular events, namely, CTCFL overexpression and enhanced immunogenic apoptosis with cell surface CRT expression and extracellular HMGB1 release, potentially leading to increased immunogenicity of tumor cells. Of interest, all these effects occurred in a limited manner in the less aggressive MDA-MB-231 cells and were not detected in the poorly invasive MCF-7 cells, thus suggesting a selective effect of these drugs on metastatic breast cancer cells.
Previous reports highlighted that CTCFL is detected at weak to moderate levels in breast cancer cell lines and the frequency of its activation is higher in metastatic tumors than primary lesions [16,38,39]. By contrast, CTCFL mRNA was not observed in eight patients with breast cancer, suggesting, in this case, a link between high methylation and absence of CTCFL expression [28]. Here, the highly invasive MDA-MB-435 cells were found to express high levels of CTCFL compared to invasively growing MDA-MB-231 cells and the nonmetastatic MCF-7 cells. Interestingly, combined treatment with apicidin and docetaxel compared to each agent alone determined a strong induction of CTCFL specifically in metastatic cancer cells. CTCFL expression is methylation dependent and it may also be affected by modification of histones [13,40]. Here, for the first time, we report that apicidin, a potent emerging HDACi, controls CTCFL expression and this activity is sustained by docetaxel. CTCFL has been reported to activate the expression of several CT genes, among which many members of the MAGE-A family and NY-ESO-1 [15,41] are already in cancer vaccine trials [42]. CTCFL represents an essential mediator of 5-aza-2′-deoxycytidine for MAGE-A1 derepression and may physically interact with the NY-ESO-1 promoter controlling its expression [14,15]. Therefore, a model has been suggested wherein CTCFL is a potential global regulator of other well-known CTs currently tested in cancer immunotherapy [43]. However, the correlation between CTCFL and other CT expression was not found in all tested human cells and tissues, implying a quite complex and conflicting CTCFL control on other CT genes in diverse cancer cells [40]. One suggested possibility is that CTCFL acts in concert with other protein partners such as Sp-1 forming a transcriptional regulatory complex that activates NY-ESO-1 in lung cancer cells [13]. Alternatively, it is conceivable that a particular isoform of CTCFL is necessary for the regulation of a defined CT so much that activation of other CT genes occurs only when a general expression of CTCFL is induced by a strong stimulus [38]. In this study, treatment of MDA-MB-435 cells with combined high doses of apicidin and docetaxel with respect to single-agent condition represents a stimulus strong enough to induce a persistent expression of CTCFL, which may then potentially favor upmodulation of both the tumor antigens NY-ESO-1 and MAGE-A1. Notably, MDA-MB-231 and MCF-7 cells did not display any significant regulation of all these genes. Given that spontaneous potent antitumor immune responses can be elicited in some patients with cancer bearing CT antigen-expressing tumors [44], our present findings indicate that apicidin, and even more its combination with docetaxel, is strongly efficacious in augmenting tumor immunogenicity of metastatic breast cancer cells. In this respect, we also observed that combined apicidin and docetaxel compared to single-agent treatment increase MHC I expression on the surface of malignant breast cancer cells. It is worth mentioning that the simultaneous increased expression of CTs and MHC I molecules by epigenetic drugs has been reported to enhance the ability of tumor cells, such as neuroblastoma and ovarian cancer cells, to be recognized by antigen-reactive CD8+ T cells [45]. Of interest, low-dose chemotherapy can elevate antigen presentation by MHC I potentiating immune sensitization of tumors [46,47].
However, HDACi as well as taxanes may act on antigen-presenting cells such as DCs by modulating their function [48–50]. In the present study, apicidin was found to induce significant up-regulation of CD86 and its combination with docetaxel was able to further modulate the increase of the maturation surface molecule CD83 on human monocyte-derived DCs. HDACi can modulate positively or negatively the expression of costimulatory signals such as CD86 on DCs depending on their maturation stage [51,52]. Here, we envisage that the combination of apicidin and docetaxel over single-agent treatment may favor the expression of DC costimulatory and maturation molecules supporting their functional activity within the inflammatory response.
Both apicidin and docetaxel are able to induce apoptosis in several types of tumor cells including breast cancer cells [8,53]. In this study, we report that in metastatic breast cancer cells the combination of apicidin and docetaxel compared to each agent alone induces the activation of conventional cell death pathways as well as signals related to immunogenic apoptosis. Recently, the concept of immunogenic cell death, relying on the capacity of a cytotoxic compound to trigger a cell death modality able to elicit cross priming by DCs of tumor antigen-specific T cells thus enhancing treatment tumoricidal activity, has emerged in some settings of chemotherapy [54,55]. This modality of cell death is distinct by “danger” and “eat me” signals such as CRT surface exposure and secretion of HMGB1 that deliver stimulatory signals to the immune system [23]. Here, we report that the highly invasive MDA-MB-435 cells treated with combined apicidin and docetaxel with respect to single-agent treatment exhibit a strong inhibition of the antiapoptotic Bcl-2 leading to an increase of the Bax/Bcl-2 ratio and a significant enhancement of cell surface CRT exposure. Moreover, in these same experimental conditions, a proteolytic maturation of caspase-8 was observed. Of interest, caspase-8 cleavage following endoplasmic reticulum stress seems to be associated with CRT exposure on cell surface in the early phase of immunogenic cell death [56]. Therefore, we envisage that apicidin and docetaxel, combined at low cytotoxic doses, may trigger cellular stress signals leading to both cell death and increased immunogenicity of metastatic cells.
HMGB1 release represents the main post-apoptotic signal of immunogenic cell death elicited by chemotherapeutic treatments [57]. Oxidized HMGB1 increases the cytotoxicity of chemotherapeutic agents or ionizing radiation and induces apoptosis through the mitochondrial pathway, whereas reducible HMGB1 induces Beclin 1-dependent autophagy and promotes cancer cell resistance to these agents [58]. Importantly, HMGB1 transmits the “injury” signal to immune, stromal, and epithelial cells, triggering inflammation [59]. In this study, combined apicidin and docetaxel over single-agent treatment determined a significant synergistic translocation of HMGB1 from nucleus into cytoplasm and subsequent release in supernatants in metastatic breast cancer cells. Although HMGB1 release has been typically associated with tumor progression and resistance to chemotherapy, its function linked to antitumor effects of immunotherapeutic treatments is becoming evident [60,61]. Very recently, a therapeutic approach based on the combination of oncolytic vaccinia virus and paclitaxel has been shown to exert synergistic antitumor effects through HMGB1 release [62]. These results suggest the hypothesis that acute HMGB1 release stimulated by the oncolytic virus, in contrast to its chronic release typically occurring in the tumor environment, may enhance the susceptibility of tumor cells to paclitaxel through activation of cellular signals, such as cell cycle checkpoints, sensitive to this agent [63]. In our model, we envisage the occurrence of a similar acute release of HMGB1 triggered by apicidin, in agreement with a previous report showing that active release of HMGB1 is determined by decreased HDAC activity [64].
In summary, we report that apicidin and docetaxel, at low toxicity doses and over single-agent treatment, stimulate direct and immunemediated antitumor effects in metastatic breast cancer cells, suggesting a rationale for using such combination in patients with advanced metastatic disease. These findings further emphasize the urgent need to develop novel combination therapies for treating malignant diseases, including metastatic breast cancer, by useful combinations of drugs functioning through complementary mechanisms of action with a manageable toxicity and avoiding phenomena of cross resistance. In this respect, our study shows that the combination of apicidin and docetaxel may represent a suitable option. These drugs, when combined, target complementary cellular pathways leading, on the one hand, to the overexpression of immunogenic tumor antigens and, on the other hand, to the induction of a type of cell death resulting in the release of immunomodulatory signals potentially able to stimulate a potent host antitumor response. In conclusion, this report discloses the cellular bases and possible molecular mechanisms supporting the rationale of combining HDACi with standard chemotherapeutic agents for the treatment of patients with metastatic breast cancer.
Supplementary Material
Acknowledgments
We acknowledge and thank F. Mattei and M. Andreotti for technical advice and Stefania Scala, G. Pascale Institute, for providing us docetaxel.
Abbreviations
- CTCFL
CTCF-like protein
- HDACi
histone deacetylase inhibitors
- CT
cancer testis
- CRT
calreticulin
- HMGB1
high-mobility group box 1 protein
- MHC I
major histocompatibility complex class I
- DCs
dendritic cells
- MTS
3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium
- GAPDH
glyceraldehyde-3-phosphate dehydrogenase
- qPCR
quantitative real-time polymerase chain reaction
- PI
propidium iodide
- Ab
antibody
- CLSM
confocal laser scanning microscopy
- DAPI
4,6-diaminidino-2-phenylindole
- MHC II
major histocompatibility complex class II
- FACS
fluorescence-activated cell sorting
- A
apicidin
- D
docetaxel
- A + D
apicidin and docetaxel combined treatment
Footnotes
This work was supported by grants from the Italian Association for Research against Cancer (AIRC) and from the Ministry of Health [www.salute.gov.it; “Progetto Integrato Oncologia” (7OAF1/4) and “Progetto ISS/NIH OF14”] to L.G.
This article refers to supplementary materials, which are designated by Figures W1 to W4 and are available online at www.neoplasia.com.
References
- 1.Jia J, Zhu F, Ma X, Cao Z, Li Y, Chen YZ. Mechanisms of drug combinations: interaction and network perspectives. Nat Rev Drug Discov. 2009;8:111–128. doi: 10.1038/nrd2683. [DOI] [PubMed] [Google Scholar]
- 2.Tyson JJ, Baumann WT, Chen C, Verdugo A, Tavassoly I, Wang Y, Weiner LM, Clarke R. Dynamic modelling of oestrogen signalling and cell fate in breast cancer cells. Nat Rev Cancer. 2011;11:523–532. doi: 10.1038/nrc3081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Higgins MJ, Baselga J. Targeted therapies for breast cancer. J Clin Invest. 2011;121:3797–3803. doi: 10.1172/JCI57152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Luu T, Chung C, Somlo G. Combining emerging agents in advanced breast cancer. Oncologist. 2011;16:760–771. doi: 10.1634/theoncologist.2010-0345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gines J, Sabater E, Martorell C, Grau M, Monroy M, Casado MA. Efficacy of taxanes as adjuvant treatment of breast cancer: a review and meta-analysis of randomised clinical trials. Clin Transl Oncol. 2011;13:485–498. doi: 10.1007/s12094-011-0686-x. [DOI] [PubMed] [Google Scholar]
- 6.Thomas S, Munster PN. Histone deacetylase inhibitor induced modulation of anti-estrogen therapy. Cancer Lett. 2009;280:184–191. doi: 10.1016/j.canlet.2008.12.026. [DOI] [PubMed] [Google Scholar]
- 7.Noh JH, Song JH, Eun JW, Kim JK, Jung KH, Bae HJ, Xie HJ, Ryu JC, Ahn YM, Wee SJ, et al. Systemic cell-cycle suppression by apicidin, a histone deacetylase inhibitor, in MDA-MB-435 cells. Int J Mol Med. 2009;24:205–226. doi: 10.3892/ijmm_00000224. [DOI] [PubMed] [Google Scholar]
- 8.Park H, Im JY, Kim J, Choi WS, Kim HS. Effects of apicidin, a histone deacetylase inhibitor, on the regulation of apoptosis in H-ras-transformed breast epithelial cells. Int J Mol Med. 2008;21:325–333. [PubMed] [Google Scholar]
- 9.Im JY, Park H, Kang KW, Choi WS, Kim HS. Modulation of cell cycles and apoptosis by apicidin in estrogen receptor (ER)-positive and -negative human breast cancer cells. Chem Biol Interact. 2008;172:235–244. doi: 10.1016/j.cbi.2008.01.007. [DOI] [PubMed] [Google Scholar]
- 10.Lai JP, Sandhu DS, Moser CD, Cazanave SC, Oseini AM, Shire AM, Shridhar V, Sanderson SO, Roberts LR. Additive effect of apicidin and doxorubicin in sulfatase 1 expressing hepatocellular carcinoma in vitro and in vivo. J Hepatol. 2009;50:1112–1121. doi: 10.1016/j.jhep.2008.12.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jones P, Altamura S, De Francesco R, Paz OG, Kinzel O, Mesiti G, Monteagudo E, Pescatore G, Rowley M, Verdirame M, et al. A novel series of potent and selective ketone histone deacetylase inhibitors with antitumor activity in vivo. J Med Chem. 2008;51:2350–2353. doi: 10.1021/jm800079s. [DOI] [PubMed] [Google Scholar]
- 12.Scanlan MJ, Gure AO, Jungbluth AA, Old LJ, Chen YT. Cancer/testis antigens: an expanding family of targets for cancer immunotherapy. Immunol Rev. 2002;188:22–32. doi: 10.1034/j.1600-065x.2002.18803.x. [DOI] [PubMed] [Google Scholar]
- 13.Kang Y, Hong JA, Chen GA, Nguyen DM, Schrump DS. Dynamic transcriptional regulatory complexes including BORIS, CTCF and Sp1 modulate NY-ESO-1 expression in lung cancer cells. Oncogene. 2007;26:4394–4403. doi: 10.1038/sj.onc.1210218. [DOI] [PubMed] [Google Scholar]
- 14.Hong JA, Kang Y, Abdullaev Z, Flanagan PT, Pack SD, Fischette MR, Adnani MT, Loukinov DI, Vatolin S, Risinger JI, et al. Reciprocal binding of CTCF and BORIS to the NY-ESO-1 promoter coincides with derepression of this cancer-testis gene in lung cancer cells. Cancer Res. 2005;65:7763–7774. doi: 10.1158/0008-5472.CAN-05-0823. [DOI] [PubMed] [Google Scholar]
- 15.Vatolin S, Abdullaev Z, Pack SD, Flanagan PT, Custer M, Loukinov DI, Pugacheva E, Hong JA, Morse H, III, Schrump DS, et al. Conditional expression of the CTCF-paralogous transcriptional factor BORIS in normal cells results in demethylation and derepression of MAGE-A1 and reactivation of other cancer-testis genes. Cancer Res. 2005;65:7751–7762. doi: 10.1158/0008-5472.CAN-05-0858. [DOI] [PubMed] [Google Scholar]
- 16.D'Arcy V, Pore N, Docquier F, Abdullaev ZK, Chernukhin I, Kita GX, Rai S, Smart M, Farrar D, Pack S, et al. BORIS, a paralogue of the transcription factor, CTCF, is aberrantly expressed in breast tumours. Br J Cancer. 2008;98:571–579. doi: 10.1038/sj.bjc.6604181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.D'Arcy V, Abdullaev ZK, Pore N, Docquier F, Torrano V, Chernukhin I, Smart M, Farrar D, Metodiev M, Fernandez N, et al. The potential of BORIS detected in the leukocytes of breast cancer patients as an early marker of tumorigenesis. Clin Cancer Res. 2006;12:5978–5986. doi: 10.1158/1078-0432.CCR-05-2731. [DOI] [PubMed] [Google Scholar]
- 18.Ghochikyan A, Mkrtichyan M, Loukinov D, Mamikonyan G, Pack SD, Movsesyan N, Ichim TE, Cribbs DH, Lobanenkov VV, Agadjanyan MG. Elicitation of T cell responses to histologically unrelated tumors by immunization with the novel cancer-testis antigen, brother of the regulator of imprinted sites. J Immunol. 2007;178:566–573. doi: 10.4049/jimmunol.178.1.566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wong ST, Goodin S. Overcoming drug resistance in patients with metastatic breast cancer. Pharmacotherapy. 2009;29:954–965. doi: 10.1592/phco.29.8.954. [DOI] [PubMed] [Google Scholar]
- 20.Grimm D, Wehland M, Pietsch J, Infanger M, Bauer J. Drugs interfering with apoptosis in breast cancer. Curr Pharm Des. 2011;17:272–283. doi: 10.2174/138161211795049723. [DOI] [PubMed] [Google Scholar]
- 21.Labi V, Grespi F, Baumgartner F, Villunger A. Targeting the Bcl-2-regulated apoptosis pathway by BH3 mimetics: a breakthrough in anticancer therapy? Cell Death Differ. 2008;15:977–987. doi: 10.1038/cdd.2008.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Locher C, Conforti R, Aymeric L, Ma Y, Yamazaki T, Rusakiewicz S, Tesniere A, Ghiringhelli F, Apetoh L, Morel Y, et al. Desirable cell death during anticancer chemotherapy. Ann N Y Acad Sci. 2010;1209:99–108. doi: 10.1111/j.1749-6632.2010.05763.x. [DOI] [PubMed] [Google Scholar]
- 23.Garg AD, Nowis D, Golab J, Vandenabeele P, Krysko DV, Agostinis P. Immunogenic cell death, DAMPs and anticancer therapeutics: an emerging amalgamation. Biochim Biophys Acta. 2010;1805:53–71. doi: 10.1016/j.bbcan.2009.08.003. [DOI] [PubMed] [Google Scholar]
- 24.Pisetsky DS, Gauley J, Ullal AJ. HMGB1 and microparticles as mediators of the immune response to cell death. Antioxid Redox Signal. 2011;15:2209–2219. doi: 10.1089/ars.2010.3865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yang D, Chen Q, Yang H, Tracey KJ, Bustin M, Oppenheim JJ. High mobility group box-1 protein induces the migration and activation of human dendritic cells and acts as an alarmin. J Leukoc Biol. 2007;81:59–66. doi: 10.1189/jlb.0306180. [DOI] [PubMed] [Google Scholar]
- 26.Bachmeier BE, Albini A, Vene R, Benelli R, Noonan D, Weigert C, Weiler C, Lichtinghagen R, Jochum M, Nerlich AG. Cell density-dependent regulation of matrix metalloproteinase and TIMP expression in differently tumorigenic breast cancer cell lines. Exp Cell Res. 2005;305:83–98. doi: 10.1016/j.yexcr.2004.12.019. [DOI] [PubMed] [Google Scholar]
- 27.Bayet-Robert M, Morvan D, Chollet P, Barthomeuf C. Pharmacometabolomics of docetaxel-treated human MCF7 breast cancer cells provides evidence of varying cellular responses at high and low doses. Breast Cancer Res Treat. 2010;120:613–626. doi: 10.1007/s10549-009-0430-1. [DOI] [PubMed] [Google Scholar]
- 28.Hines WC, Bazarov AV, Mukhopadhyay R, Yaswen P. BORIS (CTCFL) is not expressed in most human breast cell lines and high grade breast carcinomas. PLoS One. 2010;5:e9738. doi: 10.1371/journal.pone.0009738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu QJ, Gao B. Manipulation of MHC-I/TCR interaction for immune therapy. Cell Mol Immunol. 2008;5:171–182. doi: 10.1038/cmi.2008.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Torabi-Rahvar M, Bozorgmehr M, Jeddi-Tehrani M, Zarnani AH. Potentiation strategies of dendritic cell-based antitumor vaccines: combinational therapy takes the front seat. Drug Discov Today. 2011;16:733–740. doi: 10.1016/j.drudis.2011.04.010. [DOI] [PubMed] [Google Scholar]
- 31.Levitzki A, Klein S. Signal transduction therapy of cancer. Mol Aspects Med. 2010;31:287–329. doi: 10.1016/j.mam.2010.04.001. [DOI] [PubMed] [Google Scholar]
- 32.Thurn KT, Thomas S, Moore A, Munster PN. Rational therapeutic combinations with histone deacetylase inhibitors for the treatment of cancer. Future Oncol. 2011;7:263–283. doi: 10.2217/fon.11.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Miller CP, Singh MM, Rivera-Del Valle N, Manton CA, Chandra J. Therapeutic strategies to enhance the anticancer efficacy of histone deacetylase inhibitors. J Biomed Biotechnol. 2011;2011:514261. doi: 10.1155/2011/514261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Munster PN, Thurn KT, Thomas S, Raha P, Lacevic M, Miller A, Melisko M, Ismail-Khan R, Rugo H, Moasser M, et al. A phase II study of the histone deacetylase inhibitor vorinostat combined with tamoxifen for the treatment of patients with hormone therapy-resistant breast cancer. Br J Cancer. 2011;104:1828–1835. doi: 10.1038/bjc.2011.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Linares A, Dalenc F, Balaguer P, Boulle N, Cavailles V. Manipulating protein acetylation in breast cancer: a promising approach in combination with hormonal therapies? J Biomed Biotechnol. 2011;2011:856985. doi: 10.1155/2011/856985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hwang JJ, Kim YS, Kim T, Kim MJ, Jeong IG, Lee JH, Choi J, Jang S, Ro S, Kim CS. A novel histone deacetylase inhibitor, CG200745, potentiates anticancer effect of docetaxel in prostate cancer via decreasing Mcl-1 and Bcl-(XL) Invest New Drugs. 2011;30:1434–1442. doi: 10.1007/s10637-011-9718-1. [DOI] [PubMed] [Google Scholar]
- 37.Hwang JJ, Kim YS, Kim MJ, Kim DE, Jeong IG, Kim CS. Histone deacetylase inhibitor potentiates anticancer effect of docetaxel via modulation of Bcl-2 family proteins and tubulin in hormone refractory prostate cancer cells. J Urol. 2010;184:2557–2564. doi: 10.1016/j.juro.2010.07.035. [DOI] [PubMed] [Google Scholar]
- 38.de Necochea-Campion R, Ghochikyan A, Josephs SF, Zacharias S, Woods E, Karimi-Busheri F, Alexandrescu DT, Chen CS, Agadjanyan MG, Carrier E. Expression of the epigenetic factor BORIS (CTCFL) in the human genome. J Transl Med. 2011;9:213. doi: 10.1186/1479-5876-9-213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yoon SL, Kim DC, Cho SH, Lee SY, Chu IS, Heo J, Leem SH. Susceptibility for breast cancer in young patients with short rare minisatellite alleles of BORIS. BMB Rep. 2010;43:698–703. doi: 10.5483/BMBRep.2010.43.10.698. [DOI] [PubMed] [Google Scholar]
- 40.Martin-Kleiner I. BORIS in human cancers—a review. Eur J Cancer. 2012;48:929–935. doi: 10.1016/j.ejca.2011.09.009. [DOI] [PubMed] [Google Scholar]
- 41.Smith IM, Glazer CA, Mithani SK, Ochs MF, Sun W, Bhan S, Vostrov A, Abdullaev Z, Lobanenkov V, Gray A, et al. Coordinated activation of candidate proto-oncogenes and cancer testes antigens via promoter demethylation in head and neck cancer and lung cancer. PLoS One. 2009;4:e4961. doi: 10.1371/journal.pone.0004961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Geldmacher A, Freier A, Losch FO, Walden P. Therapeutic vaccination for cancer immunotherapy: antigen selection and clinical responses. Hum Vaccin. 2011;7(suppl):115–119. doi: 10.4161/hv.7.0.14573. [DOI] [PubMed] [Google Scholar]
- 43.Akers SN, Odunsi K, Karpf AR. Regulation of cancer germline antigen gene expression: implications for cancer immunotherapy. Future Oncol. 2010;6:717–732. doi: 10.2217/fon.10.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Caballero OL, Chen YT. Cancer/testis (CT) antigens: potential targets for immunotherapy. Cancer Sci. 2009;100:2014–2021. doi: 10.1111/j.1349-7006.2009.01303.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bao L, Dunham K, Lucas K. MAGE-A1, MAGE-A3, and NYESO-1 can be upregulated on neuroblastoma cells to facilitate cytotoxic T lymphocyte-mediated tumor cell killing. Cancer Immunol Immunother. 2011;60:1299–1307. doi: 10.1007/s00262-011-1037-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wan S, Pestka S, Jubin RG, Lyu YL, Tsai YC, Liu LF. Chemotherapeutics and radiation stimulate MHC class I expression through elevated interferon-beta signaling in breast cancer cells. PLoS One. 2012;7:e32542. doi: 10.1371/journal.pone.0032542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fonsatti E, Nicolay HJ, Sigalotti L, Calabro L, Pezzani L, Colizzi F, Altomonte M, Guidoboni M, Marincola FM, Maio M. Functional up-regulation of human leukocyte antigen class I antigens expression by 5-aza-2′-deoxycytidine in cutaneous melanoma: immunotherapeutic implications. Clin Cancer Res. 2007;13:3333–3338. doi: 10.1158/1078-0432.CCR-06-3091. [DOI] [PubMed] [Google Scholar]
- 48.Woan KV, Sahakian E, Sotomayor EM, Seto E, Villagra A. Modulation of antigen-presenting cells by inhibitors: implications in autoimmunity and cancer. Immunol Cell Biol. 2012;90:55–65. doi: 10.1038/icb.2011.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.John J, Ismail M, Riley C, Askham J, Morgan R, Melcher A, Pandha H. Differential effects of paclitaxel on dendritic cell function. BMC Immunol. 2010;11:14. doi: 10.1186/1471-2172-11-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Shurin GV, Tourkova IL, Kaneno R, Shurin MR. Chemotherapeutic agents in noncytotoxic concentrations increase antigen presentation by dendritic cells via an IL-12-dependent mechanism. J Immunol. 2009;183:137–144. doi: 10.4049/jimmunol.0900734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tanaka H, Matsushima H, Nishibu A, Clausen BE, Takashima A. Dual therapeutic efficacy of vinblastine as a unique chemotherapeutic agent capable of inducing dendritic cell maturation. Cancer Res. 2009;69:6987–6994. doi: 10.1158/0008-5472.CAN-09-1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pfannenstiel LW, Lam SS, Emens LA, Jaffee EM, Armstrong TD. Paclitaxel enhances early dendritic cell maturation and function through TLR4 signaling in mice. Cell Immunol. 2010;263:79–87. doi: 10.1016/j.cellimm.2010.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Montero A, Fossella F, Hortobagyi G, Valero V. Docetaxel for treatment of solid tumours: a systematic review of clinical data. Lancet Oncol. 2005;6:229–239. doi: 10.1016/S1470-2045(05)70094-2. [DOI] [PubMed] [Google Scholar]
- 54.Schiavoni G, Sistigu A, Valentini M, Mattei F, Sestili P, Spadaro F, Sanchez M, Lorenzi S, D'Urso MT, Belardelli F, et al. Cyclophosphamide synergizes with type I interferons through systemic dendritic cell reactivation and induction of immunogenic tumor apoptosis. Cancer Res. 2010;71:768–778. doi: 10.1158/0008-5472.CAN-10-2788. [DOI] [PubMed] [Google Scholar]
- 55.Haynes NM, van der Most RG, Lake RA, Smyth MJ. Immunogenic anti-cancer chemotherapy as an emerging concept. Curr Opin Immunol. 2008;20:545–557. doi: 10.1016/j.coi.2008.05.008. [DOI] [PubMed] [Google Scholar]
- 56.Panaretakis T, Kepp O, Brockmeier U, Tesniere A, Bjorklund AC, Chapman DC, Durchschlag M, Joza N, Pierron G, van Endert P, et al. Mechanisms of pre-apoptotic calreticulin exposure in immunogenic cell death. EMBO J. 2009;28:578–590. doi: 10.1038/emboj.2009.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kepp O, Galluzzi L, Martins I, Schlemmer F, Adjemian S, Michaud M, Sukkurwala AQ, Menger L, Zitvogel L, Kroemer G. Molecular determinants of immunogenic cell death elicited by anticancer chemotherapy. Cancer Metastasis Rev. 2011;30:61–69. doi: 10.1007/s10555-011-9273-4. [DOI] [PubMed] [Google Scholar]
- 58.Tang D, Loze MT, Zeh HJ, Kang R. The redox protein HMGB1 regulates cell death and survival in cancer treatment. Autophagy. 2010;6:1181–1183. doi: 10.4161/auto.6.8.13367. [DOI] [PubMed] [Google Scholar]
- 59.Andersson U, Tracey KJ. HMGB1 is a therapeutic target for sterile inflammation and infection. Annu Rev Immunol. 2011;29:139–162. doi: 10.1146/annurev-immunol-030409-101323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tang D, Kang R, Zeh HJ, III, Lotze MT. High-mobility group box 1 and cancer. Biochim Biophys Acta. 2010;1799:131–140. doi: 10.1016/j.bbagrm.2009.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Dong Xda E, Ito N, Lotze MT, Demarco RA, Popovic P, Shand SH, Watkins S, Winikoff S, Brown CK, Bartlett DL, et al. High mobility group box I (HMGB1) release from tumor cells after treatment: implications for development of targeted chemoimmunotherapy. J Immunother. 2007;30:596–606. doi: 10.1097/CJI.0b013e31804efc76. [DOI] [PubMed] [Google Scholar]
- 62.Huang B, Sikorski R, Kirn DH, Thorne SH. Synergistic anti-tumor effects between oncolytic vaccinia virus and paclitaxel are mediated by the IFN response and HMGB1. Gene Ther. 2011;18:164–172. doi: 10.1038/gt.2010.121. [DOI] [PubMed] [Google Scholar]
- 63.Zhuang W, Qin Z, Liang Z. The role of autophagy in sensitizing malignant glioma cells to radiation therapy. Acta Biochim Biophys Sin (Shanghai) 2009;41:341–351. doi: 10.1093/abbs/gmp028. [DOI] [PubMed] [Google Scholar]
- 64.Evankovich J, Cho SW, Zhang R, Cardinal J, Dhupar R, Zhang L, Klune JR, Zlotnicki J, Billiar T, Tsung A. High mobility group box 1 release from hepatocytes during ischemia and reperfusion injury is mediated by decreased histone deacetylase activity. J Biol Chem. 2010;285:39888–39897. doi: 10.1074/jbc.M110.128348. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.