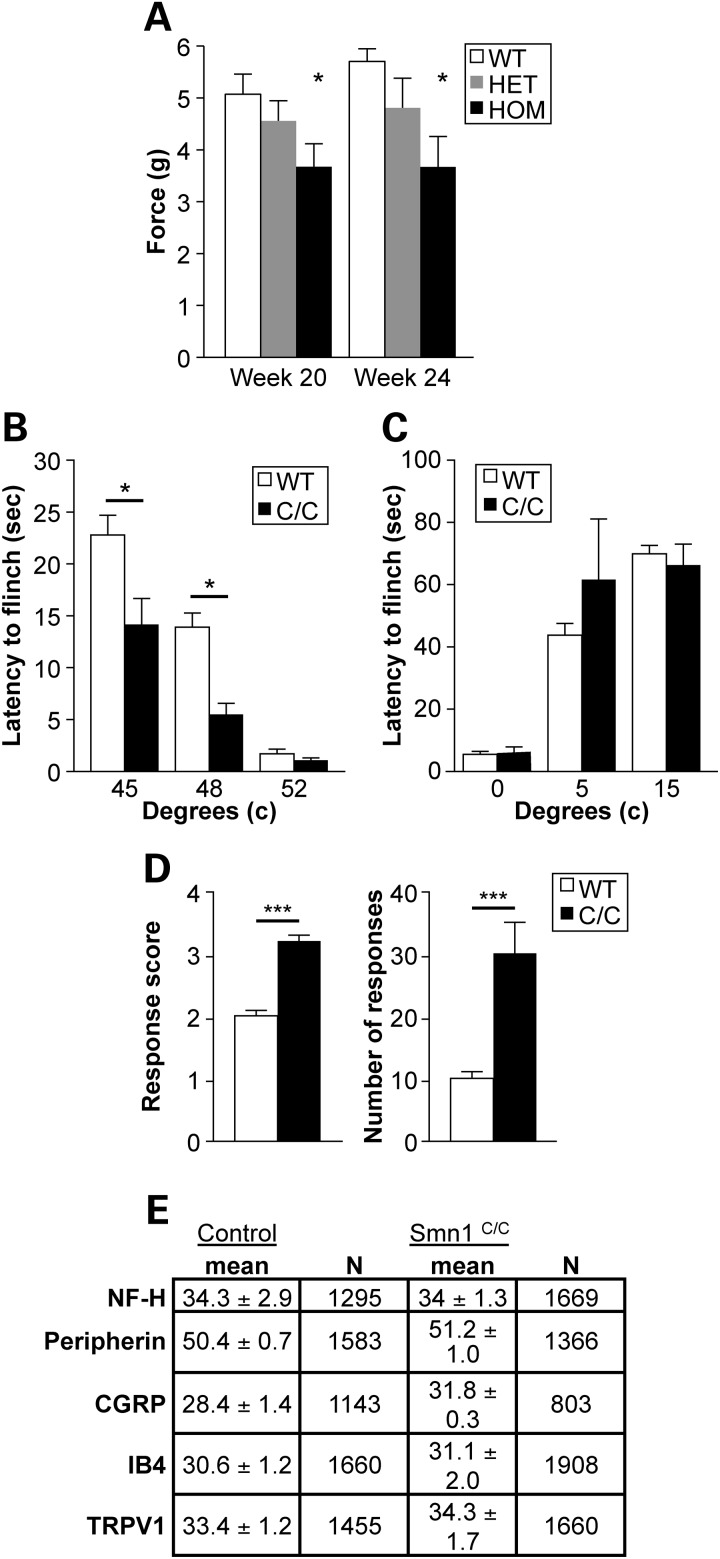
Figure 7.
Examination of heightened nociceptive responses in Smn1C/C mutant mice. (A) Homozygous Smn1C/C mutants exhibit increased nocioception at 20 and 24 weeks as assessed by von Frey at PGI. In each of these trials, paw withdrawal force was significantly lower in Smn1C/C mutant animals than both heterozygous and WT controls (P < 0.0155). (B) Smn1C/C mice exhibited an increase in sensitivity to moderately noxious heat with a significant decrease in hindpaw withdrawal latencies when the animals were placed on a metal plate heated to 45 (P < 0.01) and 48°C (P < 0.001). No differences were observed between Smn1C/C mutant animals and WT littermate controls in response to a high noxious stimulus of 52°C. (C) No differences were observed in behaviors of either genotype when exposed to noxious cold (5–15°C). (D) Smn1C/C mice exhibited robust sensitization in the evaporative cooling assay responding more vigorously with a score of 3.2 ± 0.1 compared with a score 2.0 ± 0.1 observed in WT mice, as well as in the number of responses in Smn1C/C and WT animals at 30.0 ± 5.0 and 10.0 ± 1.0 (P < 0.001), respectively. (E) Quantitation of specific markers in the somas of cutaneous nerve fibers in Smn1C/C and WT mice (n = 3/group) demonstrates no difference in the prevalence of any one fiber type. Data are the percentage of the PGP9.5 (pan neuronal marker) population of lumber dorsal root ganglion (DRG) neurons that were also immuno-reactive for a marker (NF-H, neurofilament marker of myelinated A-delta fibers; CGRP, calcitonin gene-related peptide-marker of peptidergic neurons; IB4, isolectin B4-marker of non-peptidergic neurons; TRPV1, transient receptor potential vanilloid 1 ion channel-expressed in nociceptors).