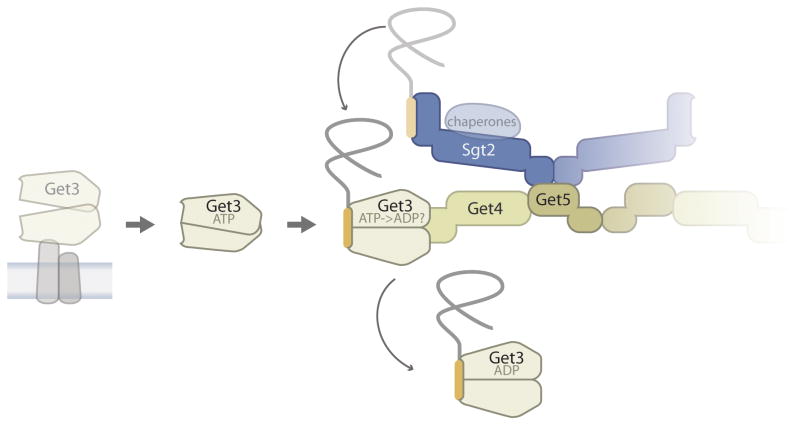
Figure 2. The pre-targeting steps of the yeast GET pathway.
ATP facilitates the release of Get3 from the membrane. Further destabilization of membrane association probably comes from competitive binding to the pre-targeting complex (containing Get4, Get5, Sgt2, and chaperones) which preferentially transfers TA proteins from Sgt2 to ATP-bound Get3 [13]. This cartoon interprets the stimulatory effect of Get4/5 on the intrinsic rate of substrate handoff [8] as an induced fit in the Get3 conformation. It is not clear if TMD binding promotes ATP hydrolysis by Get3, however, the resulting Get3-TA protein complex is shown as ADP because an assembled hydrophobic groove for substrate binding has only been visualized in the post-hydrolysis state [28,50]. Fade out of one set of Sgt2, Get5, Get4 subunits indicates that the precise stoichiometry of the pre-targeting complex is not known. For a more detailed discussion of how existing structural data fit substrate handoff models of different stoichiometries the reader is referred to a recent review [51]. The chaperones bound to the TPR domain of Sgt2 are shown as a transparent oval because their contribution to the GET pathway is unclear.