Abstract
The medulla of the adrenal gland is a neuroendocrine tissue in which catecholamine-storing chromaffin cells exist. The chromaffin cells are derived from neural crest cells and distinctly differentiated into two types of cells, epinephrine (E) (adrenaline)-storing and norepinephrine (NE) (noradrenaline)-storing cells. Using histochemical or immunostaining methods, the two types of chromaffin cells have been differentially distinguished. However, difficulties and/or drawbacks of the procedures have somewhat restricted the progress of research in differential functions of E-storing and NE-storing cells. Here, we show a new method for the differential demonstration of these two cell types. We found that mouse and rat adrenomedullary cells are heterogeneously stained with Harris hematoxylin after treatment with citrate buffer at pH 6. The cell clusters stained with hematoxylin were positive for tyrosine hydroxylase, which is an enzyme involved in catecholamine biosynthesis. Furthermore, the cell clusters were negative for phenylethanolamine-N-methyl transferase, which is an enzyme responsible for the conversion from NE to E and expresses in E-storing chromaffin cells. Moreover, we found that the cell clusters stained with hematoxylin can also be stained with nitroblue tetrazolium at pH 11, using Hopsu and Mäkinen’s method by which NE-storing chromaffin cells are stained. These observations indicate that the cytoplasm of NE-storing chromaffin cells is specifically stained with hematoxylin after treatment with citrate buffer at pH 6. This method will allow us to facilitate cell-type specific research of chromaffin cells. Indeed, this method revealed that α-synuclein selectively expresses in E-storing chromaffin cells, but not in NE-storing chromaffin cells.
Keywords: Adrenal, Medulla, Chromaffin, Epinephrine, α-Synuclein, PNMT
Introduction
The adrenal medulla is a neuroendocrine tissue that is developmentally derived from neural crest cells (Mravec 2005). In the adrenal medulla, there are many chromaffin cells that synthesize and store catecholamines in secretory vesicles called chromaffin granules (Huber et al. 2009). The chromaffin cells are innervated by nerve terminals of sympathetic preganglionic neurons originating from the intermediolateral cell column of Th4 to Th12 segments of the spinal cord via the splanchnic nerve. Upon stimulation through the nerve terminals of sympathetic preganglionic neurons, chromaffin cells release catecholamines by exocytosis, which is known as the sympathoadrenal system (Mravec 2005; Strack et al. 1988).
Within mammalian adrenal medulla, there are two distinct types of chromaffin cells secreting catecholamines directly to the blood stream. One possesses chromaffin granules storing epinephrine (E) and another possesses those storing norepinephrine (NE) (Coupland 1989). Chromaffin cells are distributed in homotypic groups according to their catecholamine phenotype, but both the ratio of E and NE phenotypes and the topographical distribution of NE cells within the medulla vary between species (Aunis and Langley 1999). For example, in the rat, E cells largely predominate (80–85 %). In human and bovine adrenal glands, about 75 % of the chromaffin cells are adrenergic (E cells). In the rabbit, the proportion of NE cells is very much smaller. The relative proportions may reflect distinct species-specific physiological requirements (Aunis and Langley 1999). In the rat, NE cells are more frequently situated at the medullary/cortical border, but this is not the case in the bovine adrenal gland (Aunis and Langley 1999).
Since these two types of chromaffin cells are distinctly differentiated, they should have different functions in addition to the release of different catecholamines. Despite extensive research, however, the differences between the two types of chromaffin cells remain unclear partly because of a difficulty in differential demonstration of E cells and NE cells (especially in the mouse medulla). Indeed, many histochemical methods do exist to distinguish the two cell types (Coupland and Hopwood 1966; Honore 1971; Hopsu and Mäkinen 1966). However, these methods, which involve special histologic procedures, have certain practical drawbacks that impair their general usefulness (Honore 1971). Silver methods have been also used, but they are elaborate and do not provide adequate histologic details (Chang and Bencosme 1968; Honore 1971; Solcia et al. 1969; Tramezzani et al. 1964). Researchers have reported immunohistochemical methods using antibodies to E and NE (Verhofstad et al. 1980) or the biosynthesizing enzyme phenylethanolamine-N-methyl transferase (PNMT) (Goldstein et al. 1971; Hokfelt et al. 1973). The immunohistochemical methods using antibodies to E or NE are useful, but regular immunostaining procedures cannot be used. Specifically, since E and NE are not immunogenic by themselves, the antibodies, which are commercially available, are generated by immunization with E or NE conjugated with glutaraldehyde and bovine serum albumin. Because of this, glutaraldehyde-fixed adrenal medulla is required for the immunostaining. The immunohistochemical method using anti-PNMT antibodies is also useful. However, the commercially available antibodies do not always cross react with PNMT of other species to the same extent, or even not cross react at all (Ubink et al. 1995). Thus, researchers have had difficulty in the differential demonstration of E cells and NE cells. This difficulty has somewhat restricted research progress in the sympathoadrenal system.
In the present study, we describe a simple and sensitive method to selectively stain NE chromaffin cells on paraffin sections of the mouse and rat adrenal medulla. This method will allow us to facilitate the research of adrenal medulla.
Materials and methods
Antibodies
Mouse monoclonal anti-tyrosine hydroxylase (TH) antibody (TH-16) and rabbit polyclonal anti-actin antibody were purchased from Sigma Chemical Company (St. Louis, MO, USA). Rabbit anti-PNMT antibody was purchased from Enzo Life Sciences (Farmingdale, NY, USA). Mouse monoclonal anti-α-synuclein antibody (4D6) and mouse monoclonal anti-synapsin IIa antibody (synapsin IIa-1) were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Goat anti-choline acetyltransferase (ChAT) antibody was purchased from Millipore (Billerica, MA, USA).
Animals
C57BL/6J mice were purchased from Jackson Laboratory (Bar Harbor, ME, USA). These mice were maintained in a facility and program accredited by the Association for Assessment and Accreditation of Laboratory Animal Care International (AAALAC) with the approval number A3307-01. The animal use protocol (#BR08-09-103) was authorized by the Institutional Animal Care and Use Committee (IACUC) of Georgia Health Sciences University.
Adrenal tissues
Twenty-week-old female mice were euthanized by CO2. The adrenal glands were dissected out and fixed in 4 % paraformaldehyde (PFA) at 4 °C for 2 weeks. Adrenal glands of SD rats (1-year-old males) were kindly provided by Dr. Gregory Liou (Georgia Health Sciences University) and fixed as well as mouse tissues. The adrenal tissues were then dehydrated and infiltrated by paraffin using an automated vacuum infiltration processor, Tissue-TeK VIP (Sakura, Torrance, CA, USA). The adrenal tissues were then embedded into paraffin blocks. The paraffin blocks were then cut into 5 µm-thick sections with a RM-2155 microtome (Leica, Buffalo Grove, IL, USA), and paraffin sections were mounted on Superfrost Plus Microscopic glass slides (Fisher Scientific, Waltham, MA, USA).
Antigen retrieval procedures
For antigen retrieval using citrate buffer, slides with adrenal sections were incubated in an oven at 60 °C for 30 min and deparaffinized in xylene and ethanol. The slides were placed in phosphate-buffered saline (PBS) for 10 min and transferred into 10 mM sodium citrate buffer (pH 6.0) and autoclaved at 121 °C for 10 min. After autoclaving, the slides were cooled for 30 min at room temperature and subsequently washed with PBS twice. For antigen retrieval using formic acid, slides with adrenal sections were treated with 97 % formic acid (Acros Organics, Fair Lawn, NJ, USA) for 5 min at room temperature and subsequently washed with PBS twice.
Immunohistochemistry
Mouse and rat adrenal sections were deparaffinized and pretreated with citrate buffer (pH 6.0) for antigen retrieval (see above). The sections were then subjected to immunohistochemical staining using the avidin–biotin–peroxidase complex method with diaminobenzidine (DAB) as the chromogen. The immunostaining was carried out using the ABC kit system (Vector, Burlingame, CA, USA). Antibodies against ChAT (diluted at 1:500), synapsin IIa (1:500), TH (1:12,800), α-synuclein (1:500), and PNMT (1:500) were used as primary antibodies. After staining, the sections were counterstained with Harris hematoxylin (cat. #HHS35-1L; Sigma) for 3 min. The sections were then dehydrated rapidly through ethanol and xylene, and mounted with VectaMount medium (Vector).
Histochemical staining of NE chromaffin cells using tetrazolium salt
We followed Hopsu and Mäkinen’s staining method (Hopsu and Mäkinen 1966) except for the buffer system. In brief, paraffin sections were deparaffinized in xylene and ethanol. The sections were rinsed with distilled water and hydrated in phosphate buffer (PB) (50 mM Na2HPO4 solution adjusted to pH 11.0). The sections were then incubated in PB containing 2 mg/ml of p-nitroblue tetrazolium chloride (NBT; Affymetrix, Santa Clara, CA, USA) at 37 °C for 90 min. The sections were washed with distilled water for 5 min and mounted with Immu-Mount (Thermo Fisher Scientific).
Double immunofluorescence staining for α-synuclein and PNMT
Mouse adrenal sections were deparaffinized and pretreated with citrate buffer (pH 6.0) for antigen retrieval (see above). The sections were then blocked with Power Block Reagent (BioGenex, Fremont, CA, USA) and 5 % horse serum, sequentially. The sections were first labeled with rabbit anti-PNMT antibody (diluted at 1:500) and mouse monoclonal anti-α-synuclein antibody 4D6 (1:500) overnight at 4 °C. After washing, the sections were labeled with Alexa fluor 594-conjugated anti-rabbit IgG for PNMT (1:1,000) and Alexa fluor 488-conjugated anti-mouse IgG for α-synuclein (1:1,000) for 1 h at room temperature. These secondary antibodies were purchased from Molecular Probes (Eugene, OR, USA). The sections were then stained with 0.1 µg/ml of 4′,6-diamidine-2-phenylindole dihydrochloride (DAPI; Roche Diagnostics, Indianapolis, IN, USA) for 7 min. Finally, the slides were analyzed by an Axio Imager M1 microscope (Zeiss, Thornwood, NY, USA). The localization of α-synuclein was shown by the green fluorescence of Alexa fluor 488. The localization of PNMT was shown by the red fluorescence of Alexa fluor 594. Their co-localization was examined by the merging of both fluorescent images.
Western blotting
We performed Western blotting as described in our recent paper (Lee and Kamitani 2011). In brief, tissue lysates were prepared from C57BL/6J mice (20-week-old females). The protein samples were treated for 30 min at 50 °C in a sample-treating solution containing 2 % SDS and 5 % β-mercaptoethanol. The samples were then loaded onto a 15 % SDS-polyacrylamide gel. After electrophoresis, proteins separated on the gel were transferred onto a polyvinylidene difluoride (PVDF) membrane (Immobilon-P; Millipore). The membrane was then treated with PBS containing 0.4 % PFA for 30 min at room temperature, followed by blocking for 1 h with 5 % skim milk. The membrane was then incubated for 1 h with a primary antibody containing 1 % skim milk. As primary antibody, mouse monoclonal anti-α-synuclein antibody 4D6 and rabbit polyclonal anti-actin antibody were used. After washing, the membrane was incubated for 1 h with a secondary antibody, horseradish peroxidase-conjugated anti-mouse IgG or anti-rabbit IgG antibody (Santa Cruz Biotechnology). After washing, protein bands on the membrane were detected by chemiluminescence method using ECL-Plus immunoblotting detection system (GE Healthcare, Piscataway, NJ, USA).
Results
Heterogenous hematoxylin counterstaining in the immunostained adrenal medulla
Adrenomedullary chromaffin cells are innervated by preganglionic cholinergic neurons (Mravec 2005; Strack et al. 1988). To investigate the cholinergic nerve terminals of the adrenal medulla, we prepared paraffin sections of PFA-fixed mouse adrenal gland. The sections were treated with citrate buffer for antigen retrieval and immunostained with antibodies to ChAT and synapsin IIa. ChAT is an enzyme responsible for acetylcholine (ACh) synthesis and thereby used as a marker for cholinergic neurons (Anglade and Larabi-Godinot 2010). Synapsin IIa is a synaptic vesicle protein that highly expresses at nerve terminals of cholinergic neurons in the adrenal medulla (Hou and Dahlstrom 1996). Therefore, we expected to visualize the cholinergic nerve terminals on adrenomedullary chromaffin cells by immunostaining of ChAT and synapsin IIa, which would give us insight into the regulation of chromaffin cells by preganglionic cholinergic neurons. As shown in Fig. 1, adrenomedullary cells were surrounded with ChAT-positive structures (panel a). Synapsin IIa was detected among adrenomedullary cells (panel b). In addition to these expected observations, we unexpectedly found that the cytoplasm of some cell clusters is strongly stained with hematoxylin (see asterisks).
Fig. 1.
Immunoperoxidase staining of the mouse adrenal medulla. Paraffin sections of PFA-fixed mouse adrenal gland were treated with citrate buffer for antigen retrieval. The sections were incubated with goat polyclonal anti-ChAT antibody (a) or mouse monoclonal antisynapsin IIa antibody (b), followed by incubation with biotinylated secondary antibody. The sections were then treated with avidin–biotin–peroxidase complex and stained using Vectastain ABC kit system. The sections were then counterstained with Harris hematoxylin and analyzed by microscopy. The localization of each antigen is shown by the brown color of DAB substrate. Counterstaining is shown by the blue color of hematoxylin. Asterisks indicate hematoxylin-positive cell clusters. Scale bars indicate 20 µm
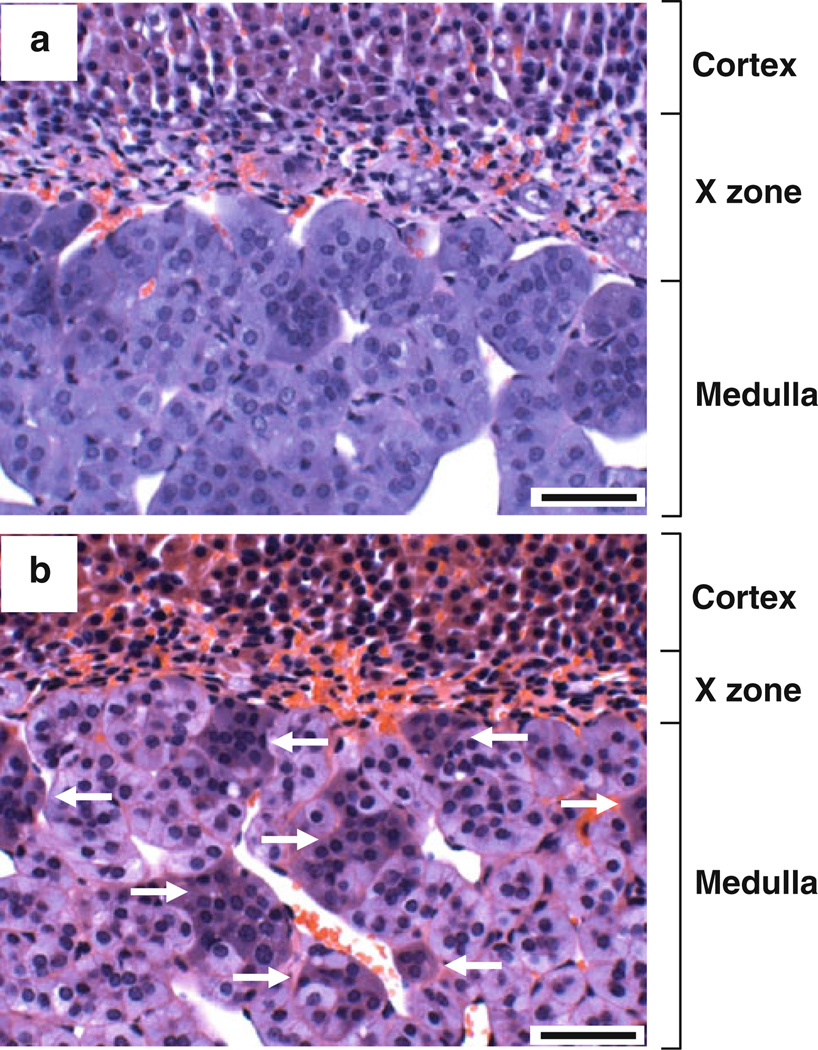
Heterogenous hematoxylin staining in the H&E-stained adrenal medulla
Using the same sections, Harris hematoxylin and eosin (H&E) staining was carried out. In this H&E staining, one section was treated with citrate buffer, but another was not. As shown in Fig. 2, the adrenal medulla was homogeneously stained with hematoxylin in the section without citrate buffer treatment (panel a). In contrast, however, the adrenal medulla was heterogeneously stained with hematoxylin in the section treated with citrate buffer (panel b). In other words, the cytoplasm of some cell clusters was strongly stained with hematoxylin (see arrows).
Fig. 2.
H&E staining of the mouse adrenal gland. Paraffin sections of PFA-fixed mouse adrenal gland were deparaffinized and stained by H&E using a standard protocol (a). Other sections were deparaffinized, treated with citrate buffer, and stained by H&E (b). Arrows indicate hematoxylin-positive cell clusters. Scale bars indicate 50 µm
Effect of citrate buffer treatment on hematoxylin staining of the adrenal medulla
The citrate buffer-treated section showed heterogenous staining by H&E (see Fig. 2b), suggesting that the antigen-retrieval procedure using citrate buffer affects hematoxylin staining in immunostaining (Fig. 1) and H&E staining (Fig. 2). To test this possibility, we simply stained paraffin sections of PFA-fixed mouse adrenal gland with Harris hematoxylin (Fig. 3). Importantly, before hematoxylin staining, the sections were untreated with antigen-retrieval solution (panel a), treated with formic acid (panel b), or treated with citrate buffer (panel c) for antigen retrieval. As expected, the regular staining without any pretreatment and the antigen retrieval with formic acid resulted in homogeneous hematoxylin staining of adrenomedullary cells (panels a and b). However, the antigen retrieval with citrate buffer led to heterogenous hematoxylin staining of adrenomedullary cells (panel c).
Fig. 3.
Effect of antigen-retrieval procedures on hematoxylin staining of the mouse adrenal medulla. Paraffin sections of PFA-fixed mouse adrenal gland were deparaffinized. The sections were stained by Harris hematoxylin without antigen retrieval (a). Other sections were treated with formic acid (b) or citrate buffer (c) for antigen retrieval and stained by Harris hematoxylin. Scale bars indicate 50 µm
Immunoreactivity of anti-TH antibody to hematoxylin-stained adrenomedullary cells
In the adrenal medulla, there are three types of cells, i.e., E or NE chromaffin cells, small intensely fluorescent (SIF) cells, equivalent to the small granule cells observed by electron microscopy, and ganglionic neurons (Aunis and Langley 1999). Which cell type is stained with hematoxylin in the adrenal medulla? Since ~20 % of adrenomedullary cells were strongly stained with hematoxylin and they were more frequently situated at the medullary/cortical border (see Figs. 1, 2), we hypothesized that NE chromaffin cells are selectively stained with hematoxylin. To test this, we first treated mouse adrenal sections with citrate buffer and immunostained chromaffin cells with an antibody to the catecholamine biosynthetic enzyme TH (Kober et al. 2010; Lloyd et al. 1986; Phillips et al. 2001), followed by counterstaining with hematoxylin. As shown in Fig. 4, the adrenal medulla was highly positive for TH (panel a). Importantly, hematoxylin-positive blue cell clusters were also stained brown with anti-TH antibody (see asterisks, panel b), suggesting that hematoxylinstained adrenomedullary cells are chromaffin cells.
Fig. 4.
Immunoperoxidase staining of the mouse adrenal medulla with anti-TH antibody. Paraffin sections of PFA-fixed mouse adrenal gland were treated with citrate buffer for antigen retrieval. The sections were incubated with mouse monoclonal anti-TH antibody, followed by incubation with biotinylated secondary antibody. The sections were then treated with avidin–biotin–peroxidase complex and stained using Vectastain ABC kit system. The sections were then counterstained with Harris hematoxylin and analyzed by microscopy. The localization of TH is shown by the brown color of DAB substrate. Counterstaining is shown by the blue color of hematoxylin. Asterisks indicate hematoxylin-positive cell clusters. Scale bars indicate 200 µm in a and 20 µm in b
Demonstration of NE chromaffin cells by histochemical method
NE chromaffin cells can be identified by many histochemical methods. Hopsu and Mäkinen’s staining is one of the classical methods. This method is based on the reduction of NBT salt at alkaline pH essentially as in the demonstration of sulfhydryl groups (Hopsu and Mäkinen 1966). To determine the cell type of hematoxylin-stained chromaffin cells, we used this method. In brief, we prepared serial (neighboring) sections of PFA-fixed mouse adrenal gland. One section was treated with citrate buffer and stained with hematoxylin. Another section was stained with NBT at pH 11 to visualize NE chromaffin cells. The staining of these two sections was then compared (Fig. 5). As shown in panel a, six cell clusters were stained with hematoxylin. Importantly, these cell clusters were also stained with NBT at the same locations (panel b). To examine the staining in more detail, we magnified the images as shown in panels c–f. Comparison between the hematoxylin staining and NBT staining (panel c vs. panel d, panel e vs. panel f) clearly revealed that hematoxylin-stained cell clusters are stained with NBT at the same locations, suggesting that hematoxylin-stained chromaffin cells are NE chromaffin cells.
Fig. 5.
Comparison of hematoxylin staining and Hopsu and Mäkinen’s staining using serial sections of the mouse adrenal medulla. A paraffin block of PFA-fixed mouse adrenal gland was cut to prepare serial (neighboring) sections. Section b was cut next to section a. Section a was deparaffinized, treated with citrate buffer, and stained by Harris hematoxylin. Section b was deparaffinized and stained with NBT in a basic buffer (pH 11) using Hopsu andMäkinen’s method. Hematoxylin-positive cell clusters (a) and NBT-stained cell clusters (b) are numbered. a is magnified in c (containing cell clusters 3 and 4) and e (containing cell clusters 3 and 6). b is magnified in d (containing cell clusters 3 and 4) and f (containing cell clusters 3 and 6). The same numbers indicate the correlated regions between a and b, c and d, and e and f. Asterisks indicate sinuses containing many red blood cells. Scale bars indicate 50 µm in a and b and 20 µm in c–f
Immunoreactivity of anti-PNMT antibody to hematoxylin-stained adrenomedullary cells
E chromaffin cells, but not NE chromaffin cells, highly express a biosynthetic enzyme PNMT that methylates NE into E. The high expression of PNMT provides the basis for a convenient immunohistochemical tool for distinguishing E cells and NE cells in tissue sections (Aunis and Langley 1999; Hokfelt et al. 1973; Kober et al. 2010; Lloyd et al. 1986; Phillips et al. 2001). Using immunohistochemistry with anti-PNMT antibody, we examined whether PNMT-positive E chromaffin cells are not stained with hematoxylin. Since the anti-PNMT antibody strongly reacted with rat PNMT but weakly with mouse PNMT, we used rat adrenal sections for this experiment. As shown in panels a and b of Fig. 6, the cytoplasm of PNMT-positive cells was not stained with hematoxylin, whereas the cytoplasm of PNMT-negative cells was stained with hematoxylin. Thus, we confirmed that the cytoplasm of NE chromaffin cells is stained with hematoxylin and that the cytoplasm of E chromaffin cells is not.
Fig. 6.
Immunoperoxidase staining of the rat adrenal medulla with anti-PNMT antibody. Paraffin sections of PFA-fixed rat adrenal gland were treated with citrate buffer for antigen retrieval. The sections were incubated with rabbit polyclonal anti-PNMT antibody, followed by incubation with biotinylated secondary antibody. The sections were then treated with avidin–biotin–peroxidase complex and stained using Vectastain ABC kit system. The sections were then counterstained with Harris hematoxylin and analyzed by microscopy (a, b). The localization of PNMT is shown by the brown color of DAB substrate. Counterstaining is shown by the blue color of hematoxylin. Scale bars indicate 20 µm
Expression level of α-synuclein in the mouse adrenal gland
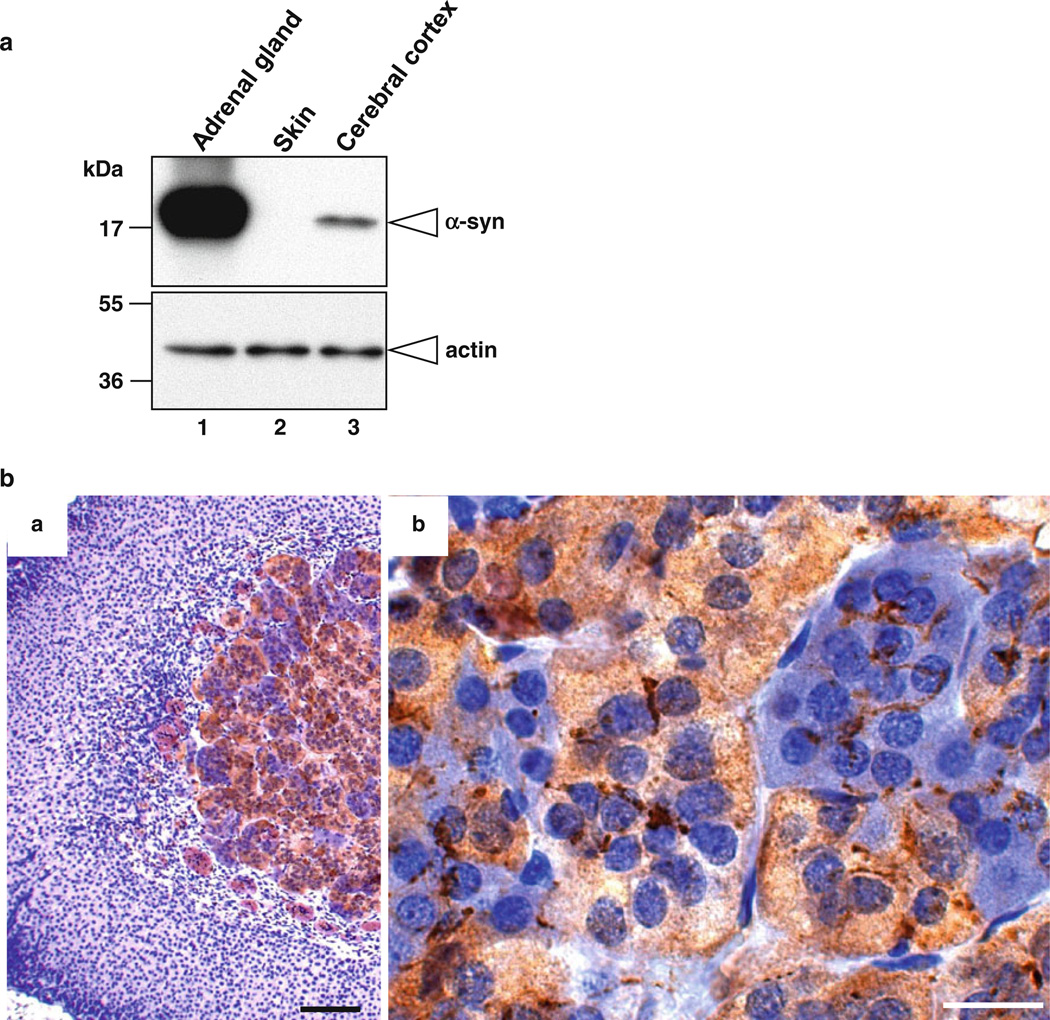
Over the past 5 years, we have investigated α-synuclein, a small soluble protein (17 kDa) that is predominantly expressed in the brain and also expressed in the spleen and kidney (Lee and Kamitani 2011). α-Synuclein predominantly localizes to synaptic vesicles and the nucleus in neurons—hence the name “synuclein” (Auluck et al. 2010; Maroteaux et al. 1988). In neurons, α-synuclein regulates the pool of synaptic vesicles through an interaction with membranes (Auluck et al. 2010). Since adrenomedullary chromaffin cells are neuronal cells and chromaffin granules are similar to synaptic vesicles, we hypothesized that α-synuclein plays a role in the adrenal medulla. To test this, we first examined the expression level of α-synuclein in the mouse adrenal gland by Western blot analysis. As positive and negative controls, we used mouse cerebral cortex (Lee and Kamitani 2011) and skin (Matsuo and Kamitani 2010), respectively. As shown in Fig. 7a, α-synuclein was not detected in the mouse skin (lane 2), whereas it was detected in the mouse adrenal gland (lane 1) and cerebral cortex (lane 3). Importantly, the expression level of α-synuclein in the adrenal gland was much higher than that in the cerebral cortex (lane 1 vs. lane 3).
Fig. 7.
Expression level and distribution of α-synuclein in the mouse adrenal gland. a Western blot analysis of α-synuclein in mouse tissues. Tissue lysates were prepared from the adrenal gland, skin, and cerebral cortex of 20-week-old C57BL/6J mice. The protein samples (~2 µg) were loaded onto a 15 % SDS-polyacrylamide gel and electrophoresed, followed by Western transfer onto a PVDF membrane. The membrane was then fixed with 0.4 % PFA for 30 min. Afterwards, the membrane was blocked and incubated with a primary antibody (anti-α-synuclein antibody 4D6 or anti-actin antibody) and a secondary antibody conjugated with horseradish peroxidase. Protein bands on the membrane were detected by ECL-Plus detection system. Molecular size markers are shown in kilodaltons (kDa). b Immunoperoxidase staining of the mouse adrenal gland with anti-α-synuclein antibody. Paraffin sections of PFA-fixed mouse adrenal gland were treated with citrate buffer for antigen retrieval. The sections were incubated with mouse monoclonal anti-α-synuclein antibody 4D6, followed by incubation with biotinylated secondary antibody. The sections were then treated with avidin–biotin–peroxidase complex and stained using Vectastain ABC kit system. The sections were then counterstained with Harris hematoxylin and analyzed by microscopy. The localization of α-synuclein is shown by the brown color of DAB substrate. Counterstaining is shown by the blue color of hematoxylin. Scale bars indicate 100 µm in a and 20 µm in b
Immunoreactivity of anti-α-synuclein antibody to hematoxylin-stained adrenomedullary cells
We next determined the distribution of α-synuclein-positive cells in the adrenal gland. For this purpose, we applied the above described hematoxylin staining to the immunohistochemical staining of α-synuclein. Briefly, mouse adrenal sections were treated with citrate buffer and immunostained with an anti-α-synuclein antibody, followed by counterstaining with hematoxylin. As shown in Fig. 7b, the adrenal medulla was positive for α-synuclein (panel a). Interestingly, the expression of α-synuclein was detected in hematoxylin-negative chromaffin cells, whereas it was not detected in hematoxylin-positive chromaffin cells (see panel b). It should be noted that nerve terminals surrounding chromaffin cells were also positive for α-synuclein. These observations suggest that α-synuclein selectively expresses in E chromaffin cells. Thus, hematoxylin staining of citrate buffer-treated sections appears to be useful for differential investigation of E and NE chromaffin cells in the adrenal medulla.
α-Synuclein expression in PNMT-positive chromaffin cells
As described above, PNMT is expressed in E chromaffin cells, but not in NE chromaffin cells. To make sure that α-synuclein is selectively expressed in E chromaffin cells, we double immunostained the adrenal medulla with mouse anti-α-synuclein antibody and rabbit anti-PNMT antibody and analyzed by immunofluorescence microscopy. As shown in Fig. 8, α-synuclein was expressed in some cell clusters, but not in others (panel a), which is consistent with the staining result shown in panel b of Fig. 7b. PNMT was also expressed in some cells clusters (panel b, Fig. 8). Importantly, the merged image revealed that PNMT-positive cell clusters are positive for α-synuclein and that PNMT-negative cell clusters are negative for α-synuclein (panel c, Fig. 8). These observations clearly indicate that α-synuclein is selectively expressed in E chromaffin cells.
Fig. 8.
Double immunofluorescence staining of the mouse adrenal medulla with anti-α-synuclein antibody and anti-PNMT antibody. Paraffin sections of PFA-fixed mouse adrenal gland were treated with citrate buffer for antigen retrieval. The sections were then double-immunostained with mouse monoclonal anti-α-synuclein antibody (4D6) and rabbit polyclonal anti-PNMT antibody. After washing, the sections were labeled with Alexa Fluor 488-conjugated anti-mouse IgG antibody and Alexa Fluor 594-conjugated anti-rabbit IgG antibody. The sections were then analyzed by fluorescence microscopy. The localization of α-synuclein is shown by the green fluorescence of Alexa Fluor 488 (a). The localization of PNMT is shown by the red fluorescence of Alexa Fluor 594 (b). Both images are merged in c. A scale bar indicates 20 µm
Discussion
In the fields of histology and pathology, tissues are usually fixed with aldehyde fixatives such as formaldehyde (formalin) and PFA and embedded with paraffin. In immunohistochemistry using such tissue sections, however, researchers often had difficulty in detection of antigens until antigen-retrieval methods were developed. The most common method for antigen retrieval is the heating of tissue sections in citrate buffer at pH 6 (Cuevas et al. 1994; Odagiri et al. 2012; Shi et al. 1993; Tanji et al. 2010). Using this method, we recently immunostained the adrenal medulla, followed by nuclear counterstaining with hematoxylin, and found that the cytoplasm of NE-storing chromaffin cells is selectively stained with hematoxylin only when the antigen-retrieval procedure is carried out in citrate buffer.
To distinguish between E and NE chromaffin cells, many histochemical and immunostaining methods have been reported. In comparison with these methods, our method has several advantages. First, the procedure is simple and easily carried out. Second, the sensitivity and specificity for the detection of NE chromaffin cells are high. Third, the cost of the staining is very low. Fourth, since all nuclei can be stained with hematoxylin, additional nuclear counterstaining is not required. Fifth, after staining, sections can be dehydrated with ethanol, cleared with xylene, and mounted with regular mounting solution. Therefore, aqueous mounting solution is not required, which results in the better quality of staining results. Sixth, double staining can easily be done using immunohistochemical staining and our hematoxylin staining. Thus, our method is very useful to differentially investigate E and NE chromaffin cells. Indeed, using an immunohistochemical approach with this method, we have successfully demonstrated the selective expression of α-synuclein in E chromaffin cells.
In conclusion, although we do not know the mechanism by which NE chromaffin cells are stained with hematoxylin after citrate buffer treatment, this method will enhance the progress in studies of the biology of adrenomedullary chromaffin cells.
Acknowledgments
We thank Dr. William E. Rainey for helpful discussion and Dr. Gregory Liou for kindly providing rat adrenal glands. This work was supported in part by National Institutes of Health Grant R01AG024497 (to T.K.).
Abbreviations
- E
Epinephrine
- NE
Norepinephrine
- PNMT
Phenylethanolamine-N-methyl transferase
- TH
Tyrosine hydroxylase
- ChAT
Choline acetyltransferase
- PFA
Paraformaldehyde
- DAB
Diaminobenzidine
- PB
Phosphate buffer
- PBS
Phosphate-buffered saline
- NBT
p-Nitroblue tetrazolium chloride
- Ach
Acetylcholine
- H&E
Hematoxylin and eosin
References
- Anglade P, Larabi-Godinot Y. Historical landmarks in the histochemistry of the cholinergic synapse: perspectives for future researches. Biomed Res. 2010;31:1–12. doi: 10.2220/biomedres.31.1. [DOI] [PubMed] [Google Scholar]
- Auluck PK, Caraveo G, Lindquist S. α-Synuclein: membrane interactions and toxicity in Parkinson’s disease. Annu Rev Cell Dev Biol. 2010;26:211–233. doi: 10.1146/annurev.cellbio.042308.113313. [DOI] [PubMed] [Google Scholar]
- Aunis D, Langley K. Physiological aspects of exocytosis in chromaffin cells of the adrenal medulla. Acta Physiol Scand. 1999;167:89–97. doi: 10.1046/j.1365-201x.1999.00580.x. [DOI] [PubMed] [Google Scholar]
- Chang WW, Bencosme SA. Selective staining of secretory granules of adrenal medullary cells by silver methenamine: a light and electron microscopic study. Can J Physiol Pharmacol. 1968;46:745–747. doi: 10.1139/y68-116. [DOI] [PubMed] [Google Scholar]
- Coupland RE. The natural history of the chromaffin cell—twenty-five years on the beginning. Arch Histol Cytol. 1989;52(Suppl):331–341. doi: 10.1679/aohc.52.suppl_331. [DOI] [PubMed] [Google Scholar]
- Coupland RE, Hopwood D. The mechanism of the differential staining reaction for adrenaline- and noradrenaline-storing granules in tissues fixed in glutaraldehyde. J Anat. 1966;100:227–243. [PMC free article] [PubMed] [Google Scholar]
- Cuevas EC, Bateman AC, Wilkins BS, Johnson PA, Williams JH, Lee AH, Jones DB, Wright DH. Microwave antigen retrieval in immunocytochemistry: a study of 80 antibodies. J Clin Pathol. 1994;47:448–452. doi: 10.1136/jcp.47.5.448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein M, Fuxe K, Hokfelt T, Joh TH. Immunohistochemical studies on phenylethanolamine-N-methyltransferase, dopa-decarboxylase and dopamine-hydroxylase. Experientia. 1971;27:951–952. doi: 10.1007/BF02135767. [DOI] [PubMed] [Google Scholar]
- Hokfelt T, Fuxe K, Goldstein M, Joh TH. Immunohistochemical localization of three catecholamine synthesizing enzymes: aspects on methodology. Histochemie. 1973;33:231–254. doi: 10.1007/BF00274236. [DOI] [PubMed] [Google Scholar]
- Honore LH. A light microscopic method for the differentiation of noradrenaline- and adrenaline-producing cells of the rat adrenal medulla. J Histochem Cytochem. 1971;19:483–486. doi: 10.1177/19.8.483. [DOI] [PubMed] [Google Scholar]
- Hopsu V, Mäkinen EO. Two methods for the demonstration of noradrenaline-containing adrenal medullary cells. J Histochem Cytochem. 1966;14:434–435. doi: 10.1177/14.5.434. [DOI] [PubMed] [Google Scholar]
- Hou XE, Dahlstrom A. Synaptic vesicle proteins in cells of the sympathoadrenal lineage. J Auton Nerv Syst. 1996;61:301–312. doi: 10.1016/s0165-1838(96)00100-2. [DOI] [PubMed] [Google Scholar]
- Huber K, Kalcheim C, Unsicker K. The development of the chromaffin cell lineage from the neural crest. Auton Neurosci. 2009;151:10–16. doi: 10.1016/j.autneu.2009.07.020. [DOI] [PubMed] [Google Scholar]
- Kober AK, Aoyama M, Sugita S. Immunohistochemical localization of catecholamine biosynthetic enzymes in the adrenal gland of the domestic fowl (Gallus domesticus) Poult Sci. 2010;89:1709–1715. doi: 10.3382/ps.2009-00588. [DOI] [PubMed] [Google Scholar]
- Lee BR, Kamitani T. Improved immunodetection of endogenous α-synuclein. PLoS ONE. 2011;6 doi: 10.1371/journal.pone.0023939. e23939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lloyd RV, Sisson JC, Shapiro B, Verhofstad AA. Immunohistochemical localization of epinephrine, norepinephrine, catecholamine- synthesizing enzymes, and chromogranin in neuroendocrine cells and tumors. Am J Pathol. 1986;125:45–54. [PMC free article] [PubMed] [Google Scholar]
- Maroteaux L, Campanelli JT, Scheller RH. Synuclein: a neuron-specific protein localized to the nucleus and presynaptic nerve terminal. J Neurosci. 1988;8:2804–2815. doi: 10.1523/JNEUROSCI.08-08-02804.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuo Y, Kamitani T. Parkinson’s disease-related protein, α-synuclein, in malignant melanoma. PLoS ONE. 2010;5 doi: 10.1371/journal.pone.0010481. e10481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mravec B. A new focus on interoceptive properties of adrenal medulla. Auton Neurosci. 2005;120:10–17. doi: 10.1016/j.autneu.2005.04.005. [DOI] [PubMed] [Google Scholar]
- Odagiri S, Tanji K, Mori F, Kakita A, Takahashi H, Kamitani T, Wakabayashi K. Immunohistochemical analysis of Marinesco bodies, using antibodies against proteins implicated in the ubiquitin-proteasome system, autophagy and aggresome formation. Neuropathology. 2012 doi: 10.1111/j.1440-1789.2011.01267.x. (in press) [DOI] [PubMed] [Google Scholar]
- Phillips JK, Dubey R, Sesiashvilvi E, Takeda M, Christie DL, Lipski J. Differential expression of the noradrenaline transporter in adrenergic chromaffin cells, ganglion cells and nerve fibres of the rat adrenal medulla. J Chem Neuroanat. 2001;21:95–104. doi: 10.1016/s0891-0618(00)00113-7. [DOI] [PubMed] [Google Scholar]
- Shi SR, Chaiwun B, Young L, Cote RJ, Taylor CR. Antigen retrieval technique utilizing citrate buffer or urea solution for immunohistochemical demonstration of androgen receptor in formalin-fixed paraffin sections. J Histochem Cytochem. 1993;41:1599–1604. doi: 10.1177/41.11.7691930. [DOI] [PubMed] [Google Scholar]
- Solcia E, Sampietro R, Capella C. Differential staining of catecholamines, 5-hydroxytryptamine and related compounds in aldehyde-fixed tissues. Histochemie. 1969;17:273–283. doi: 10.1007/BF00309872. [DOI] [PubMed] [Google Scholar]
- Strack AM, Sawyer WB, Marubio LM, Loewy AD. Spinal origin of sympathetic preganglionic neurons in the rat. Brain Res. 1988;455:187–191. doi: 10.1016/0006-8993(88)90132-1. [DOI] [PubMed] [Google Scholar]
- Tanji K, Kamitani T, Mori F, Kakita A, Takahashi H, Wakabayashi K. TRIM9, a novel brain-specific E3 ubiquitin ligase, is repressed in the brain of Parkinson’s disease and dementia with Lewy bodies. Neurobiol Dis. 2010;38:210–218. doi: 10.1016/j.nbd.2010.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tramezzani JH, Chiocchio S, Wassermann GF. A technique for light and electron microscopic identification of adrenalin- and noradrenalin-storing cells. J Histochem Cytochem. 1964;12:890–899. doi: 10.1177/12.12.890. [DOI] [PubMed] [Google Scholar]
- Ubink R, Lange W, Verhofstad A. Simultaneous immunoenzymatic staining of catecholamines, catecholamine-biosynthesizing enzymes, and bromodeoxyuridine in adrenal medullary cells of the rat. J Histochem Cytochem. 1995;43:39–46. doi: 10.1177/43.1.7822762. [DOI] [PubMed] [Google Scholar]
- Verhofstad AA, Steinbusch HW, Penke B, Varga J, Joosten HW. Use of antibodies to norepinephrine and epinephrine in immunohistochemistry. Adv Biochem Psychopharmacol. 1980;25:185–193. [PubMed] [Google Scholar]