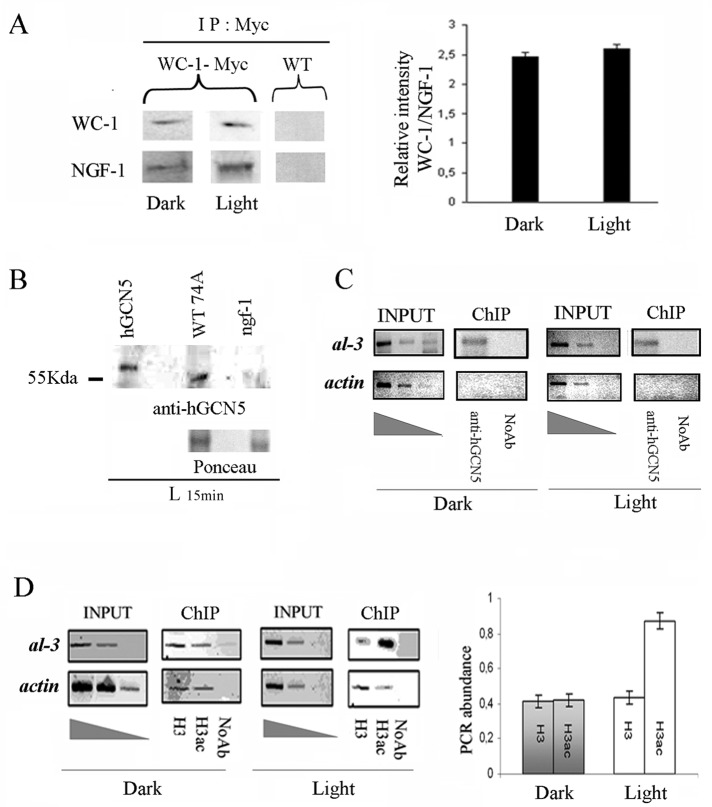
FIGURE 1:
NGF-1 physically interacts with WC-1 in dark and light conditions. (A) Myc WC-1 was immunoprecipitated (IP) using a commercial anti-Myc antibody from 400 μg of total protein extracts from Neurospora mycelia grown for 3 d in the dark before harvesting (Dark) or exposed to saturating white light for 5 min and then returned to the dark for 20 min before harvesting (Light 25 min). Precipitated WC-1 and coprecipitated NGF-1 were detected with anti-Myc and anti-hGCN5, respectively. As control of the specificity of the signal obtained, the same experiment was performed on the WT strain without the Myc epitope (left). Quantification of the ratio between the relative optical density of WC-1 and NFG-1 bands from three independent experiments under Dark and Light conditions (right). The data are the mean ± SEM from three independent experiments. (B) Western blot with anti-hGCN5 against hGCN5 purified protein, Neurospora WT, and ngf-1 strains confirms the absence of cross-hybridization of the heterologous antibody. Ponceau staining was used to check the presence of similar protein amounts on the membrane. (C) ChIP assay on Neurospora mycelia grown for 3 d in the dark before harvesting (Dark) or exposed to saturating white light for 5 min and then returned to the dark for 20 min before harvesting (Light 25 min). DNA immunoprecipitated with anti-hGCN5 antibody was amplified for the al-3 light-responsive promoter region. A sequence for actin was used as negative control. INPUT, PCR on chromatin samples before immunoprecipitation. ChIP, PCR from anti-hGCN5 IP samples. Noab, PCR from samples without antibody. (D) ChIP analysis as described in C was performed with anti–histone H3 (H3) and anti–acetylated histone H3 (H3ac; left). Quantification of the PCR abundance for the al-3 promoter was calculated as described in Materials and Methods (right). The data are the mean ± SEM from three independent experiments.