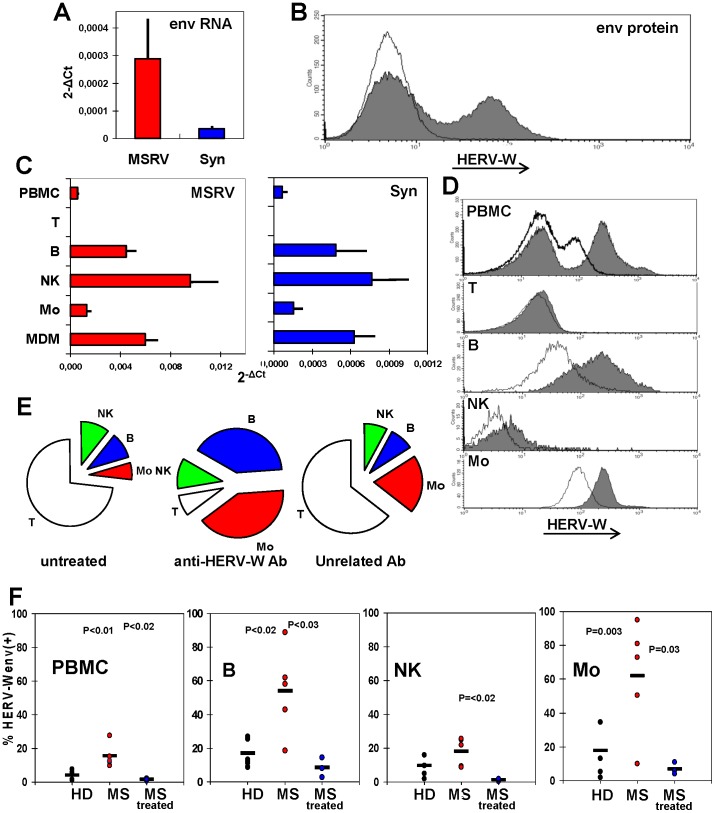
Figure 2. Basal expression of MSRVenv/ syncytin-1/HERV-Wenv in U87-MG astrocytes and PBMC subsets. A.
Detection of MSRVenv and syncytin-1 mRNAs of U87-MG cells, by real time RT-PCR (means of three experiments run in duplicate, calculated by the 2−ΔCt method; bars indicate standard deviation. B. Flow cytometry evaluation of the HERV-Wenv protein on U87-MG plasma membrane. Shaded histograms: HERV-Wenv-specific staining; open histograms: isotype control. C. MSRVenv and Syncytin-1 mRNA expression on PBMC from MSRV(+) donors as such, and after immunobeads separation in CD3+T, CD19+ B, CD56+/CD19−/CD3− NK and CD19−/CD3–/CD56− monocyte subsets, and subsequent monocyte differentiation into MDM (bars indicate standard deviation). D. Flow cytometry evaluation of the HERV-Wenv protein on the membrane of PBMC from a representative MSRV(+) donor in toto and after sorting of the CD3+T, CD19+ B, CD56+/CD19−/CD3− NK and CD19−/CD3−/CD56− monocyte subsets. Shaded histograms: HERV-Wenv-specific staining; open histograms: isotype control. E. Cell populations distribution of PBMC from a representative MSRV(+) donor before, and after capture by magnetic beads charged with anti-HERV-Wenv or with an unrelated isotype antibody. The unprocessed PBMC and the cells retained by the immunobeads were sorted for T, B, NK and monocyte markers. The unretained cells were also analysed (not shown). F. Presence of surface HERV-Wenv protein in blood cells from five MSRV(+) HD, five untreated MS patients and three MS patients under effective therapy, evaluated by flow cytometry in PBMC in toto and after sorting of the CD3+T, CD19+ B, CD56+/CD19−/CD3− NK and CD19−/CD3−/CD56− monocyte subsets. Each dot represents an individual; horizontal bars represent the means. HERV-Wenv positivity of CD3+T cells was <2% for all samples (not shown).