Figure 5.
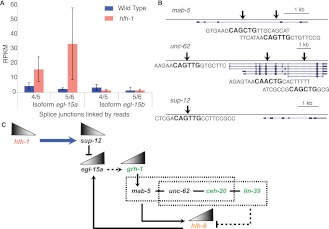
hlh-1 negatively regulates the NSM transcription factor hlh-8. (A) Differential splicing of different isoforms of egl-15 (a and b) is hlh-1-regulated in BWM. Splicing isoform b, specific to BWM, is unaffected by the mutation, while isoform a, specific to NSM, is up-regulated specifically in the mutant muscle. (B) HLH-1 binding sites local to the genes mab-5, unc-62, and sup-12 are shown, with arrows representing the ChIP-seq peaks. Each gene contains an E-box motif characteristic of hlh-1 occupancy. sup-12 is the only one whose expression depends on hlh-1, but the other genes may be regulated in part by hlh-1. (C) sup-12 depends on HLH-1 binding for expression (blue arrow). The mRNA-binding protein SUP-12 inhibits the splicing variant EGL-15a (Kuroyanagi et al. 2007). In the absence of hlh-1, grh-1 is up-regulated and may be controlled (dashed line) by egl-15a (Zhong and Sternberg 2006) to regulate mab-5 (Venkatesan et al. 2003). In turn, MAB-5 competes with LIN-39 to interact with CEH-20 and UNC-62 in some cells to effect target expression or repression (Liu et al. 2006; Jiang et al. 2009; Potts et al. 2009). They may act on hlh-8, which is known in some cells to depend on ceh-20 and mab-5 (Kenyon 1986; Liu et al. 2006; Jiang et al. 2009). Therefore, in the absence of hlh-1, sup-12 decreases—leading to an increase in egl-15a and grh-1. GRH-1 and EGL-15a work to activate the MAB-5/UNC-62/CEH-20 Hox/Pbx complex to up-regulate the normally repressed hlh-8. This pathway is supported by the appearance of grh-1, ceh-20, and lin-39 (shown in green) in the synthetic PAT screen as being integral for muscle formation.