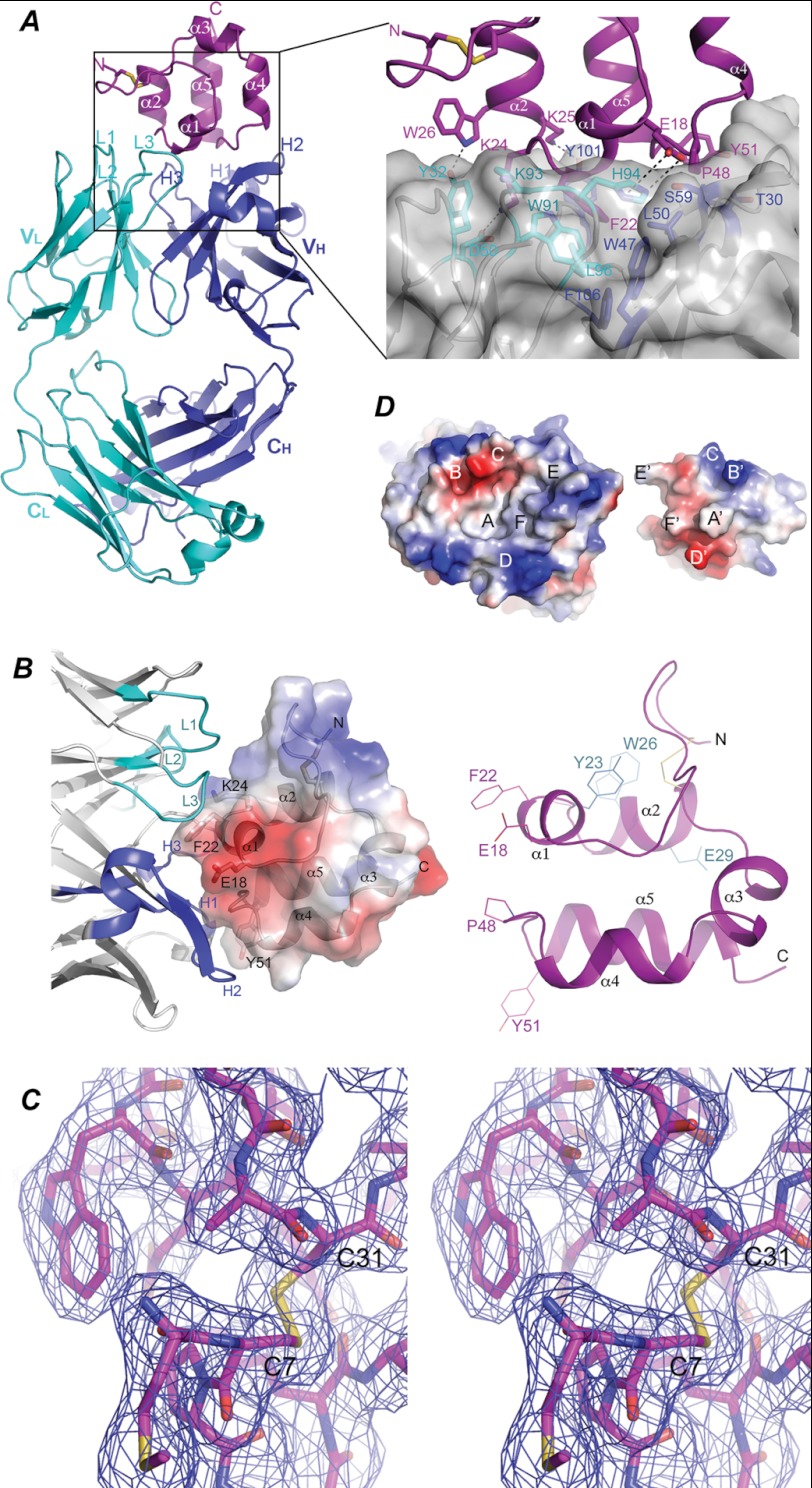
FIGURE 3.
Molecular interactions between mesothelin and Fab. A, shown is a ribbon diagram of the structure of the Msln-(7–64)-Fab complex. The mesothelin fragment is colored in magenta. The N-terminal disulfide bond is illustrated as sticks in yellow. The Fab light chain and heavy chain are colored in cyan and blue, respectively. Variable and constant domains of the light chain (VL and CL) and the heavy chain (VH and CH) are indicated. CDRs of the light chain are labeled as L1, L2, and L3, respectively, and those of heavy chain are labeled H1, H2, and H3, respectively. Inset, shown is an enlarged view of the hydrophobic and aromatic-aromatic interactions between Phe-22 of mesothelin and Fab. Residues involving in interacting with Phe-22 of mesothelin are labeled. B, a close up view of the structure of Msln-(7–64) is shown. Right panel, the five α-helices are labeled as α1, α2, α3, α4, and α5, sequentially, from the N to C terminus. Residues important for interacting with Fab are labeled in magenta, and those for interacting with CA-125 are shown in cyan. Left panel, a semitransparent electrostatic potential surface is plotted covering the structure of the fragment with positive and negative potentials in blue and red, respectively. CDRs for the light chain are colored in cyan, and those for the heavy chain are in blue. C, a stereoscopic pair shows a region of mesothelin in stick model around the disulfide bond between Cys-7 and Cys-31. Overlapping this model is a 2Fo − Fc map contoured at 1.0 σ level showing the quality of electron density. D, the electrostatic potential surfaces show the chemical and shape complementarity between the interacting mesothelin and Fab. The mesothelin molecule is intentionally flipped by 180° to expose the interacting surfaces, revealing a concave surface on the side of Fab and a convex one on the mesothelin side. The two surfaces are in perfect shape and chemical complementation. Complementing pairs on both surfaces are labeled as A-A′, B-B-, etc.