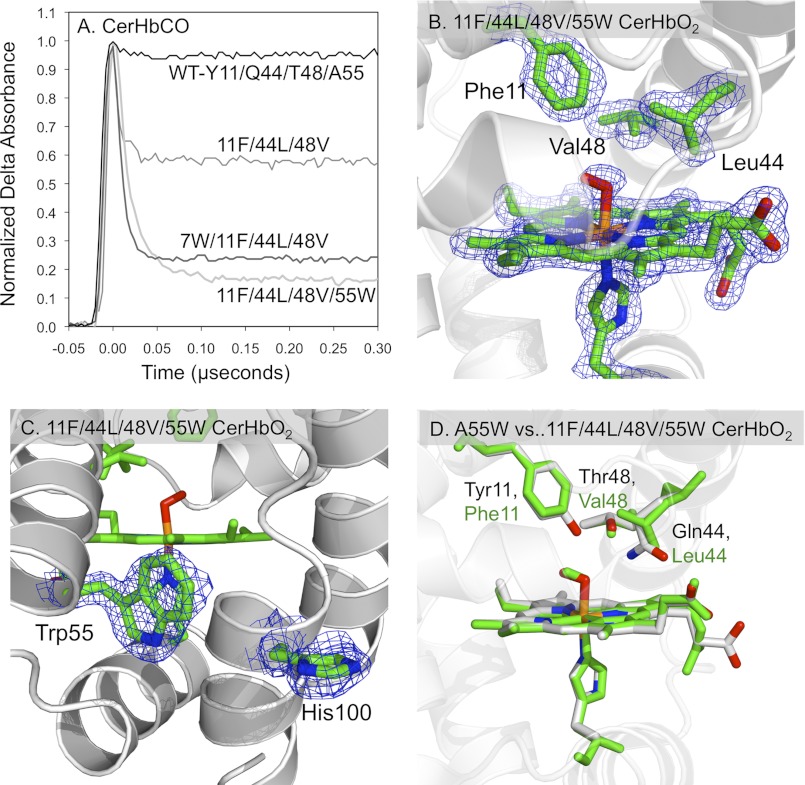
FIGURE 6.
Effects of an apolar active site on the structural and geminate recombination properties of CerHbCO. A, geminate rebinding in multiple mutants of CerHbCO with an apolar distal pocket containing Y11F/Q44L/T48V mutations at the B10, E7, and E11 helical positions, respectively, and Trp replacements at the Val-7(B6) and Ala-55(E18) tunnel positions. B, 2Fo − Fc electron density maps cutoff at 1.5 σ for the heme group, axial ligands, Phe-11(B10), Leu-44(E7), and Val-48(E11) in Y11F/Q44L/T48V/A55W CerHbO2 (PDB code 4F68). As shown, all three side chains are very well defined. C, 2Fo − Fc electron density maps for Trp-55(E18) cutoff at 1.5 σ and His-100(H11) cutoff at 1.0 σ in Y11F/Q44L/T48V/A55W CerHbO2 (PDB code 4F68). The side chain for His-100 is less well defined compared with the indole ring of Trp-55. D, comparison of the active sites of A55W and Y11F/Q44L/T48V/A55W CerHbO2. The Cα atoms of the structures for A55W (PDB code 2VYY) and quadruple CerHbO2 mutant (PDB code 4F68) were superimposed in PyMOL. The heme and active site side chains of the Y11F/Q44L/T48V/A55W CerHbO2 mutant are drawn as green sticks, whereas the heme and active site polar side chains of A55W CerHbO2 are drawn in CPK colors, with carbon, oxygen, nitrogen, and iron atoms being gray, red, blue, and orange, respectively. Positions 11, 44, 48, and 55 correspond to the B10, E7, E11, and E18 helical locations in the globin fold.