FIGURE 2.
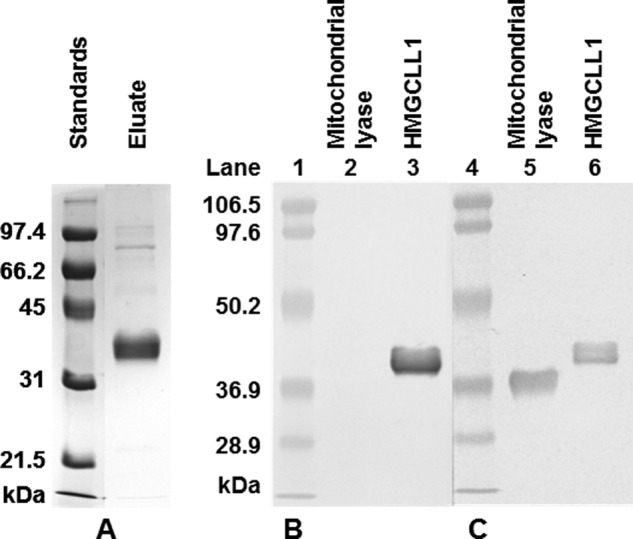
Purification of HMGCLL1 and specificity of antibody against HMGCLL1. A, left panel, HMGCLL1 protein was expressed in P. pastoris. 5 μg of protein from a Ni-Sepharose eluate were subjected to electrophoresis on a 10% SDS gel. Protein bands were detected using a Coomassie stain. Molecular weight markers (left lane) include (highest to lowest): phosphorylase b, 97.4 kDa; bovine serum albumin, 66.2 kDa; ovalbumin, 45 kDa; carbonic anhydrase, 31 kDa; and trypsin inhibitor, 21.5 kDa. Right panel, duplicate samples of purified human mitochondrial HMG-CoA lyase and wild-type HMGCLL1 (1 μg each) were subjected to electrophoresis on an SDS gel and transblotted to nitrocellulose. The duplicate halves were separated and treated individually with either (B) rabbit anti-HMGCLL1 antibody (whole serum) or (C) rabbit anti-avian HMG-CoA lyase antibody (whole serum). The blots were incubated with alkaline phosphatase-conjugated secondary antibody. Protein bands were colorimetrically detected using 5-bromo-4-chloro-3-indolyl phosphate/nitro blue tetrazolium. Prestained molecular mass markers (Bio-Rad low range; lot number 310001516) include: phosphorylase b, 106.5 kDa; bovine serum albumin, 97.6 kDa; ovalbumin, 50.2 kDa; carbonic anhydrase, 36.9 kDa; trypsin inhibitor, 28.9 kDa.
