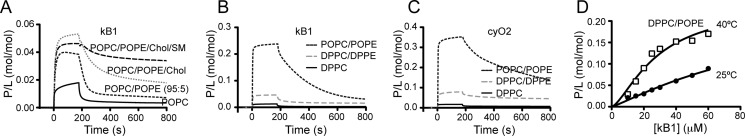
FIGURE 5.
Importance of the PE-phospholipid environment for cyclotide binding. A, SPR sensorgrams obtained at 25 °C upon injection of 30 μm of kB1 for 180 s over POPC, POPC/POPE (90:5), POPC/POPE/Chol (62:5:33), and POPC/POPE/Chol/SM (22:5:33:40) at 25 °C. Dissociation was followed for 600 s. The response units were converted to P/L to normalize the response to the total amount of lipid deposited in the chip and to the number of peptide moles that bind to the lipid bilayer. Comparison of POPC versus POPC/POPE (95:5) reveals that 5% of POPE is enough to improve the binding of kB1 to the membrane. Including 33% of Chol further improves the affinity, and the dissociation rate is decreased. The introduction of 40% SM decreases the dissociation rate of kB1 from the lipid membrane. B, SPR sensorgrams obtained at 25 °C upon injection of 100 μm of kB1 or (C) cyO2 for 180 s over DPPC, DPPC/DPPE (80:20), or POPC/POPE (80:20) deposited on L1 chip. Dissociation was monitored for 600 s after the injection stopped. The sensorgrams show only weak binding to the gel-like DPPC bilayer. Although an increase in affinity is seen with DPPC/DPPE membranes, the amount of peptide binding to this mixture in the gel phase is much lower than for POPC/POPE in the fluid-disordered phase. D, binding of kB1 to DPPC/POPE (80:20) at 25 °C and 40 °C. P/L is plotted versus peptide concentration. An increase in binding with temperature confirms that kB1 prefers more fluid membranes over solid membranes.