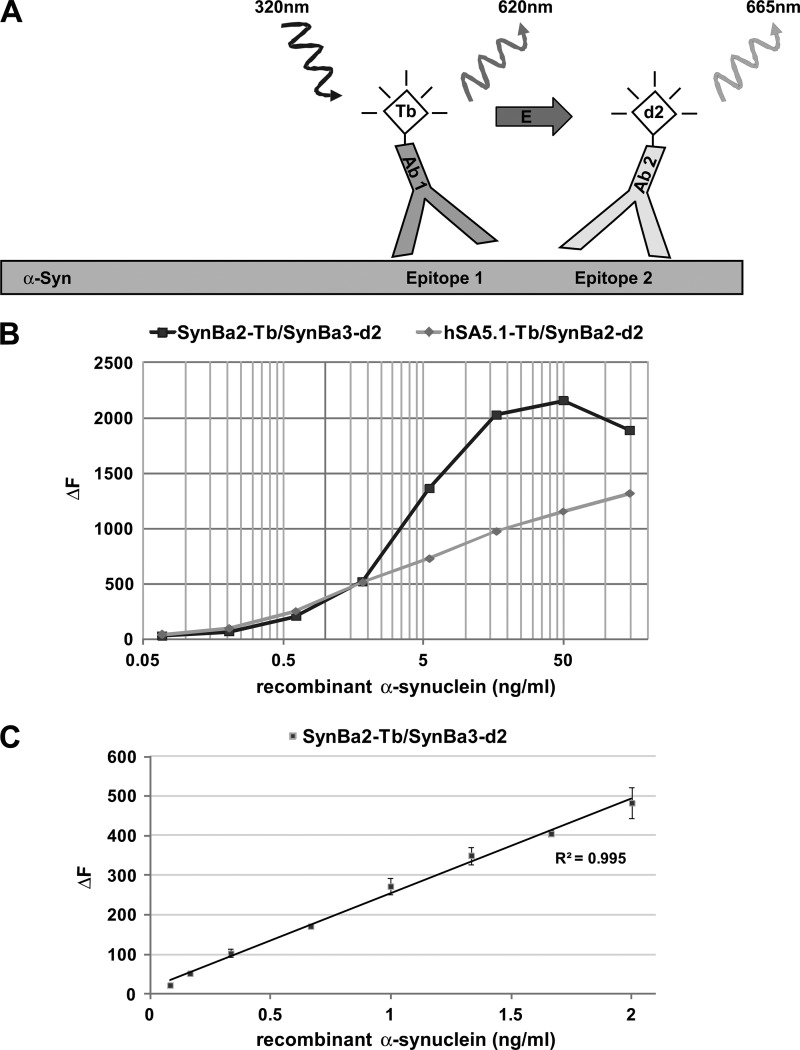
FIGURE 1.
Design of TR-FRET immunoassay and dynamic range for recombinant α-synuclein. A, shown is representation of the TR-FRET assay principle. Coincident, in-solution binding of two fluorophore-labeled antibodies to unique epitopes on α-synuclein generates the TR-FRET signal. Excitation of the Tb donor fluorophore causes both analyte-independent fluorescence (620 nm) and non-radiative energy transfer to the d2 acceptor fluorophore. The latter TR-FRET signal (665 nm) is dependent on the presence of α-synuclein. B, shown is broad range dilution of α-synuclein and detection by the antibody pairs SynBa2-Tb/SynBa3-d2 and hSA5.1-Tb/SynBa2-d2. Recombinant α-synuclein was serially diluted in lysis buffer (PBS containing 1% Triton X-100) starting from 150 ng/ml in polystyrene 384-well microtiter plates followed by TR-FRET detection (average of duplicates). C, TR-FRET detection at low α-synuclein concentrations is shown. Recombinant α-synuclein was fractionally diluted in polystyrene plates to concentrations between 2 and 0.02 ng/ml followed by detection with SynBa2-Tb/SynBa3-d2.