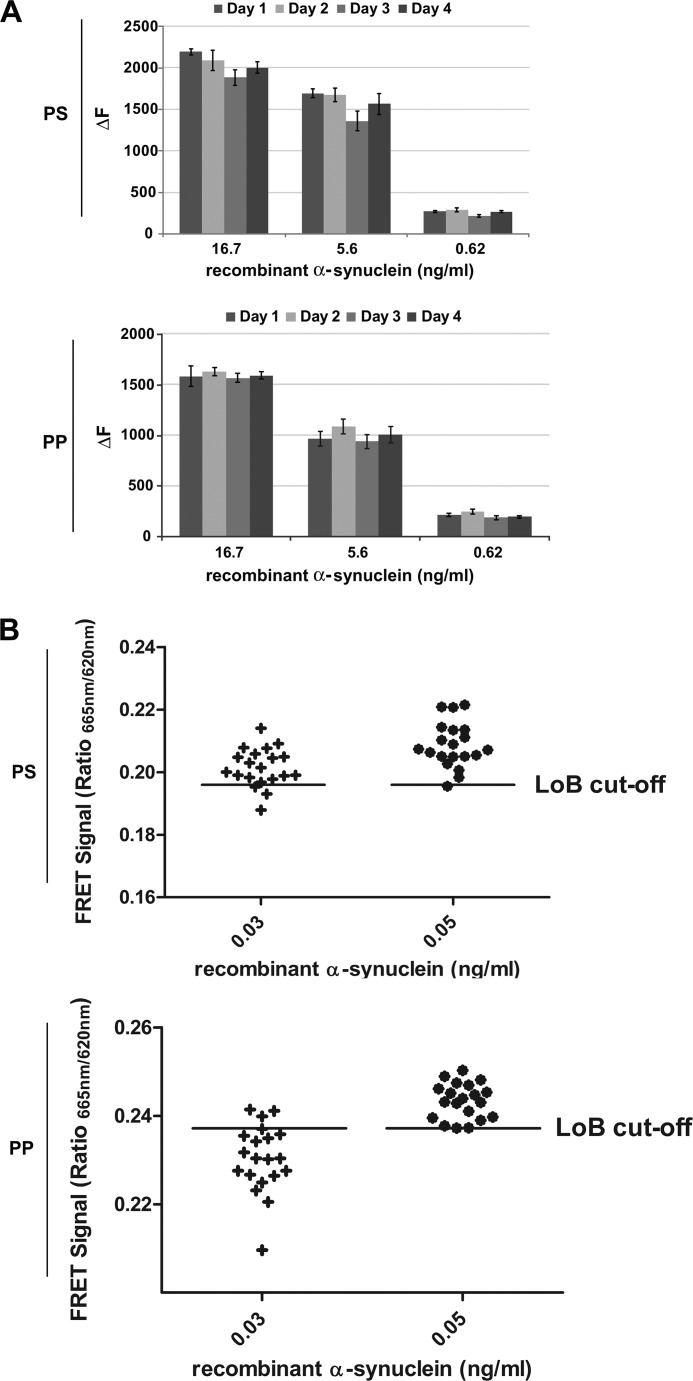
FIGURE 2.
Characterization of α-synuclein TR-FRET assay robustness and LoD. A, interassay variability assessment in PS and PP plates is shown. Recombinant α-synuclein was serially diluted (quadruplicates) freshly on each of four independent days in 384-well microtiter plates, as indicated, followed by TR-FRET detection. Fluorescence measurements were taken 20 h after antibody addition. B, the α-synuclein limits of detection in polystyrene and polypropylene plates were determined according to the procedure outlined by the Clinical Laboratory and Standards Institute (29). Recombinant α-synuclein was prepared at low concentrations corresponding to putative limits of detection as calculated from pilot experiments. Twenty replicates of each sample were then measured, and the LoD was accepted for concentrations at which at least 95% of the samples yielded TR-FRET signals above the LoB cut-off (determined from 40 blank replicates).