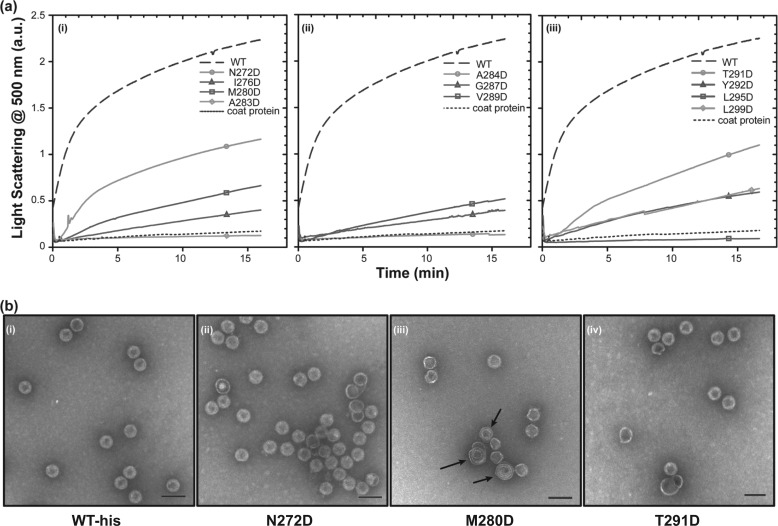
FIGURE 2.
Kinetic analysis of in vitro procapsid assembly. a, monomeric coat protein (0.5 mg/ml) was mixed with scaffolding protein variants (0.5 mg/ml) in buffer. The progress of procapsid polymerization was monitored by light scattering. Reactions for each of the scaffolding protein mutants are shown as follows: (i) mutations in helix 1, (ii) mutations in the β1-turn and its periphery, and (iii) mutations in helix 2. b, micrographs of selected in vitro assembled procapsids made with different scaffolding protein variants in 45 mm NaCl as follows: (i) WT scaffolding protein, (ii) N272D, (iii) M280D, and (iv) T291D scaffolding proteins. No partial procapsids are readily evident in these micrographs because normal PCs are generated in 45 mm NaCl. The arrows in b (iii) point to spiral structures. The scale bar represents 100 nm. All micrographs were taken at ×68,000 magnification.