FIGURE 7.
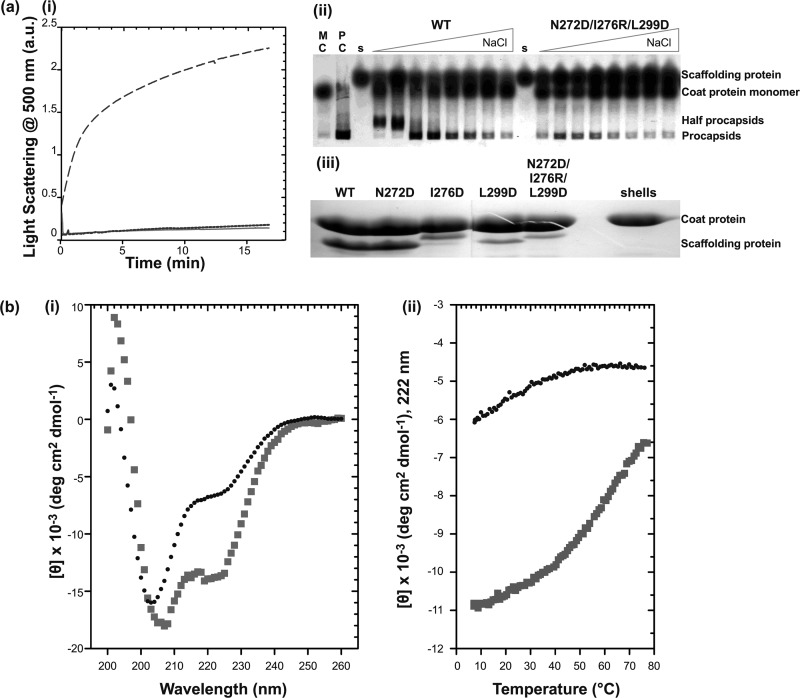
Replacement of hydrophobic interactions in the HTH core with salt bridges. a, activity assay of full-length N272D/I276R/L299D scaffolding protein. (i) the effect of the N272D/I276R/L299D mutations on the in vitro assembly as measured by light scattering. Samples of 0.5 mg/ml (— —) WT or (solid line) N272D/I276R/L299D scaffolding proteins were mixed with 0.5 mg/ml of coat protein and monitored for 16 min. As negative control, assembly of 0.5 mg/ml of the (— —) coat protein with no scaffolding protein was also measured. (ii) salt titration of PC assembly at various concentrations of NaCl (0, 5, 15, 30, 45, 60, 75, and 100 mm). Lanes with monomeric coat protein (MC), in vivo assembled procapsids (PC), and each scaffolding protein are indicated. (iii) shell refilling assay of the ability of scaffolding protein to bind empty procapsid shells. Solutions of 0.33 mg/ml of procapsid shells and 0.22 mg/ml of scaffolding protein variants were incubated overnight at room temperature. Refilled procapsids were purified by centrifugation through a 20% sucrose cushion and the proteins in the pellet were visualized with 12.5% SDS-PAGE. b, CD measurements of WT and N272D/I276R/L299D mutant 6Xhis-Δ1–237/T265W scaffolding proteins fragments: (i) CD spectra of 0.5 mg/ml of WT (squares) and N272D/I276R/L299D (circles) fragments. (ii) Measurement of molar ellipticity signal at 222 nm with increasing temperature showing WT (squares) and N272D/I276R/L299D (circles).