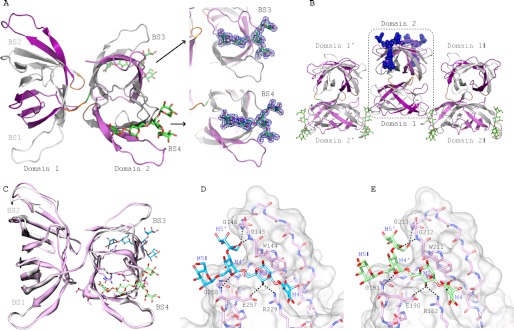
FIGURE 5.
Crystal structures of α3,α6-mannopentaose-bound MBHA. A, ribbon representation of α3,α6-mannopentaose-bound MBHA structure. The five β-strands from the first and second sequence repeat in the first and second domain of MBHA are colored in white and purple, and in gray and light magenta, respectively. The connecting amino acids between the sequence repeats (between strands β5 and β6 and strands β15 and β16) and between the first and second domain in MBHA are colored orange. The two bound α3,α6-mannopentaose molecules in binding sites 3 and 4, located in the second domain, are depicted in green stick representation, with oxygen atoms shown in red. The electron density contoured at 1.0σ enclosing the molecular model of α3,α6-mannopentaose (or Manα(1–3)[Manα(1–3)[Manα(1–6)]Manα(1–6)]Man pentasaccharide) in stick representation in binding sites 3 and 4 of MBHA is colored blue. B, arrangement of α3,α6-mannopentaose-bound MBHA depicting two neighboring molecules in the crystal. The color scheme and secondary structure depiction are similar to that in A. C, superposition of ligand-free and α3,α6-mannopentaose-bound MBHA structures in ribbon representation. The bound α3,α6-mannopentaose binding sites 3 and 4 are depicted in cyan and light green stick representation, with oxygen atoms shown in red. D and E, details of the interaction network between α3,α6-mannopentaose and MBHA in binding sites 3 and 4. The protein is depicted in both surface and stick representations, with the bound α3,α6-mannopentaose only in stick representation. Residues that are in direct contact with the carbohydrate are the following: Trp144, Gly145, Gly146, Arg229, Glu257, and Gly258 in binding site 3 (D) and Trp211, Gly212, Gly213, Arg162, Glu190, and Gly191 in binding site 4 (E). Intermolecular hydrogen bonds are indicated by black dashed lines. The same color scheme as in C is used for the carbohydrate. Protein residues are labeled by single-letter code, and the sugar rings of the carbohydrate are labeled according to standard nomenclature.