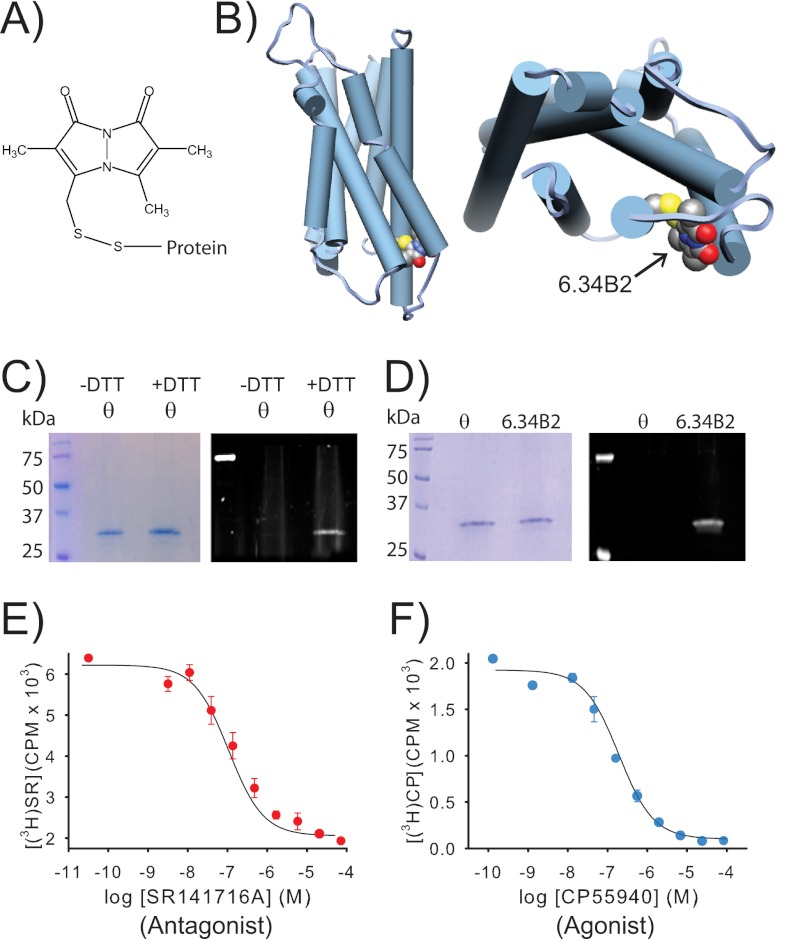
FIGURE 1.
A purified CB1 receptor, specifically labeled with a bimane fluorophore at site 6.34 on TM6, can still bind agonist and antagonist. A, the structure of PDT-bimane. B, a model of CB1 showing the probe covalently attached at A342C (C6.34) on the cytoplasmic face of TM6. C, Coomassie Blue-stained SDS-PAGE gel (left) of purified minimal-cysteine construct mutant θ (which contains only Cys-257 and Cys-264). Ultraviolet irradiation of the same gel (right), before staining, shows that θ does not react with PDT-bimane unless it is first reduced with DTT, prior to labeling (note the bimane fluorescence in the DTT treated sample). This result provides direct chemical evidence that Cys-257 and Cys-264 are in a disulfide bond in CB1. D, left, a Coomassie Blue-stained SDS-PAGE gel showing that the immunopurified CB1 mutants θ and A342C/θ can be purified to homogeneity. Right, in-gel fluorescence of the same gel before Coomassie Blue staining shows only that the A342C/θ mutant exhibits fluorescence, indicating that the bimane is uniquely and specifically covalently attached at A342C in TM6. E and F, the same purified, detergent-solubilized, bimane-labeled A342C/θ from D is functional, as indicated by its ability to bind antagonist, SR141716A (SR) (E), and agonist, CP55940 (CP) (F), in solution. Further details are provided under “Experimental Procedures.” Error bars indicate range.