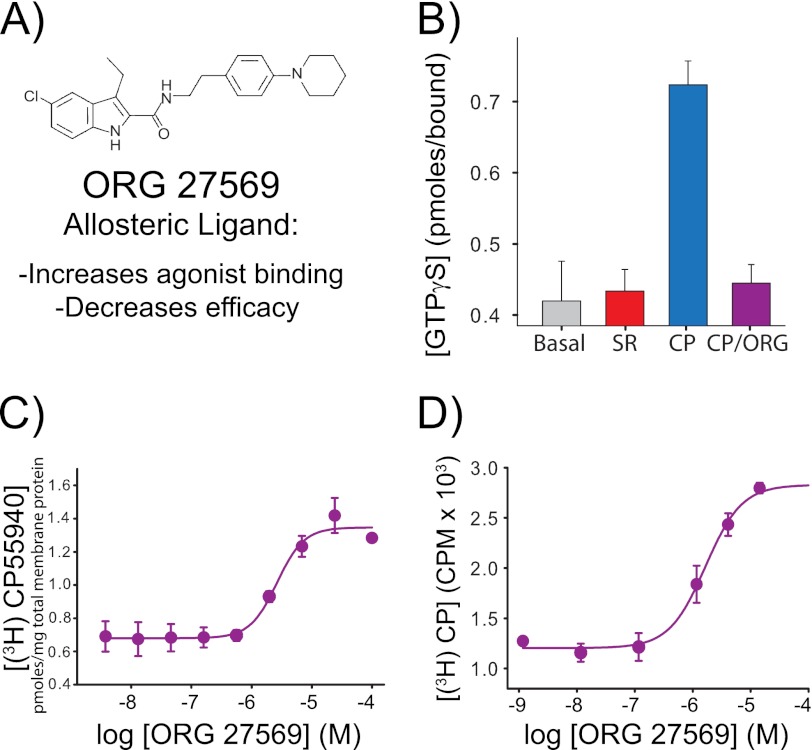
FIGURE 3.
The allosteric CB1 modulator Org 27569 enhances agonist (CP55940) binding yet inhibits agonist-induced G protein activation. A, molecular structure of the allosteric ligand Org 27569. B, the purified, detergent-solubilized bimane-labeled CB1 mutant A342C/θ is functionally active; it stimulates G protein activation upon the addition of agonist (10 μm CP55940 (CP), blue) as measured by [35S]GTPγS binding to purified Gαiβγ. In contrast, no agonist ligands (gray bar) or antagonist ligands (10 μm SR141716A (SR), red bar) show less GTPγS binding. G protein activation is blocked when Org 27569 is added along with agonist (10 μm Org 27569 + 10 μm CP55940 (CP/Org), purple). Note that Org 27569 does not block agonist binding; instead, it actually increases agonist binding ([3H]CP55940) to CB1. C and D, we observed this phenomenon for CB1 mutant A342C/θ in membranes (EC50 for CP55940 binding enhancement = 2.7 ± 0.7 μm) (C) and in a bimane-labeled, detergent-solubilized, purified form (EC50 for CP55940 binding enhancement = 1.9 ± 0.6 μm) (D). All radioactive binding studies are representative of two independent experiments performed in duplicate, shown as mean ± S.E. The specific equilibrium binding of [3H]CP55940 in C and D were determined in the presence of various concentrations of Org 27569 when compared with saturating amounts of cold CP55940. The EC50 values were determined by fitting a variable slope sigmoidal dose-response function to the combined respective data sets, and errors were determined from least squares fitting. See “Experimental Procedures” for more details.